Biology 107 Carbon and Molecular Diversity August 31, 2005
advertisement

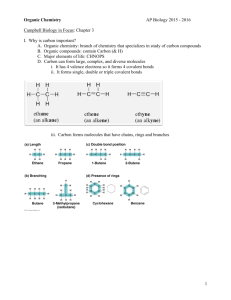
Biology 107 Carbon and Molecular Diversity August 31, 2005 Carbon Student Objectives: As a result of this lecture and the assigned reading, you should understand the following: 1. Role of carbon in life's diversity - next to water, compounds containing carbon are the most common substances in living organisms. 2. The enormous variety of carbon-based molecules is because a carbon atom has 4 outer shell electrons in a shell that holds 8. 3. Compounds with the same molecular formula but different structure are called isomers. Carbon 4. The unique properties of an organic compound depend not only on its carbon skeleton, but also on certain groups of atoms that are covalently linked to the skeleton. These groups of atoms are called functional groups, the name reflecting the fact that these parts of the organic molecules usually are involved in chemical reactions. See Figure 4.10 in Campbell and Reece. 5. Most of these functional groups are polar, because their oxygen or nitrogen atoms are highly electronegative. The polarity tends to make compounds containing these groups hydrophilic, and therefore soluble in water - a necessary condition for their roles in water-based life. Note that many biological molecules have two or more functional groups (e.g., amino acids - contain at least one carboxyl as well as one amino group). Carbon 6. Organic macromolecules are polymers created through dehydration synthesis reactions that chemically link the specific monomers together with covalent bonds. Polymers are broken down through hydrolysis reactions. 7. It is the variety in polymers that accounts for the uniqueness of each organism; the monomers used to make polymers are essentially universal throughout the biological realm. Types of Bonds Intramolecular Intermolecular Covalent Hydrogen Ionic Van der Waals Metallic Ionic Attractions Examples of Bond Strengths Bond Type Bond Length (nm) Bond Strength in Water (kcal/mole) Covalent 0.15 90 Ionic 0.25 3 Hydrogen 0.30 1 Van der Waals (per atom) 0.35 0.1 Organic Molecules Contain Carbon Valence Shells of Atoms Most Commonly Found in Organic Molecules Majors Types of Isomers 1. Structural isomers (different covalent arrangements) 2. Geometric isomers (differ around double bond) 3. Enantiomers (mirror-images that differ around an asymmetric, chiral, atom) What is Chirality? Immanuel Kant, 1783 “The glove of one hand cannot be used on the other” (S) “sinister” LEFT (R) “rectus” RIGHT ENANTIOMERS Enantiomers have identical physical and chemical properties. EXCEPT Ability to rotate the plane of polarized light and Rate of reaction and interaction with other chiral compounds and environments Importance of Chirality O N H O O N N H O H O N OH H (-)-Propranolol Different activities? N O O (-)-Thalidomide H O H (+)-Thalidomide N H HO H (+)-Propranolol Same activity different potency CHIRALITY AND DRUG ACTION Why do enantiomers have the potential for exhibiting different pharmacodynamic and/or pharmacokinetic properties? Primary Functional Groups Functional Groups C O-H 1. Hydroxyl H 2. Carbonyl C C O aldehyde O C C ketone C Functional Groups 3. Carboxyl O O H C C O C + + H C O 4. Amino H C N H + H+ H + C N H H Functional Groups 5. Sulfhydral C S-H 6. Phosphate O H C O P O O H O C O P O O + 2 H+ Slight Differences in Functional Groups May Have Dramatic Functional Effects Biological Molecules Usually Have More Than One Functional Group Dehydration Synthesis Reactions - Additions Hydrolysis Reaction - Removals Polymer Synthesis and Breakdown Reactions Synthesis of complex molecules from simpler molecules (anabolism) by dehydration synthesis reactions Break down of complex molecules to simpler molecules (catabolism) by hydrolysis reactions Why so many different metabolic enzymes in cells?