Carbon, Isomers, Functional Groups
advertisement

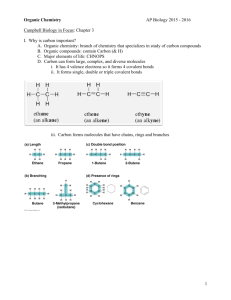
CARBON AND THE MOLECULAR DIVERSITY OF LIFE Chapter 4 How well do you know the carbon atom? • Number of protons ______ • Number of neutrons ______ • Number of electrons ______ • Atomic Number ______ • Atomic Mass ______ • Valence Electrons ______ • Electrons needed to fill valence shell ______ • Type of bond carbon atoms readily form ____________ Valences of the major elements of organic molecules • Hydrogen ______ • Oxygen ______ • Nitrogen ______ Structural formula for CO2 and CO(NH2)2 Carbon Dioxide Urea Naming Hydrocarbons: __________ __________ __________ __________ __________ __________ II. Hydrocarbons: naming conventions Roots/Prefixes (2C stands for two carbons) 1 C - ____meth__ 2 C - ____eth____ 3 C - ____prop___ 4 C - ____but____ 5 C - ____pent____ Butane Butane 6 C - ____hex____ 7 C - ____hept___ 8 C - ____oct____ 9 C - ____non____ 10 C - ___dec____ Butane Hydrocarbon Naming Conventions, Formulas and Examples Series of Hydrocarbon -ending Formula to determine # of H atoms * Bonding Alkane ANE Cn H2n+2 Single Bonds Only Alkene ENE Cn H2n One Double Bond C Alkyne YNE Cn H2n-2 One Triple Bond C * Where “n” is the number of carbon atoms C C Hydrocarbons: Name the molecule: C6H12O6 Isomers: • Compounds with the same molecular formula but different structures and properties. Isomers: Structural Isomers: • Have different covalent arrangements of their atoms Geometric Isomers: • Have the same covalent arrangements but differ in spatial arrangements Enantiomers: • Isomers that are mirror images of each other • The middle carbon is called an asymmetric carbon because it is attached to four different atoms or groups of atoms How many asymmetric carbons are in this molecule? a b c d e ONE!!! Enantiomers: • Enantiomers are important in the pharmaceutical industry • Two enantiomers of a drug may have different effects • Differing effects of enantiomers demonstrate that organisms are sensitive to even subtle variations in molecules Enantiomers: Functional Groups: • Functional groups are the components of organic molecules that are most commonly involved in chemical reactions • The number and arrangement of functional groups give each molecule its unique properties Functional Groups: • The seven functional groups that are most important in the chemistry of life: • Hydroxyl group • Carbonyl group • Carboxyl group • Amino group • Sulfhydryl group • Phosphate group • Methyl group Hydroxyl: What it looks Like… What it Makes…… alcohol Example(s): Functional Properties: Is polar Can form hydrogen bonds with water molecules Carbonyl: What it looks Like… What it Makes…… Aldehyde – end Ketone - middle Example(s): Functional Properties: A ketone and aldehyde may be structural isomers These two groups are found in sugars giving rise to two major types of sugars Carboxyl: What it looks Like… What it Makes…… Carboxylic Acid Example(s): Functional Properties: Has acidic properties (is a source of hydrogen ions) because the covalent bond between the oxygen and hydrogen is so polar Amino: What it looks Like… What it Makes…… Amine Example(s): Functional Properties: Acts as a base; can pick up an H+ from the surrounding solution Ionized, with a charge of 1+, under cellular conditions Sulfhydryl: What it looks Like… What it Makes…… Thiol Example(s): Functional Properties: Two sulfhydryl groups can react forming a covalent bond. This “crosslinkage” helps stabilize protein structure. Phosphate: What it looks Like… What it Makes…… Organic Phosphate Example(s): Functional Properties: Has the potential to react with water, releasing energy Methyl: What it looks Like… What it Makes…… Methylated Compounds Example(s): Functional Properties: Addition of a methyl group to DNA, or to molecules bound to DNA, affects expression of genes.