Human Biochemistry
advertisement

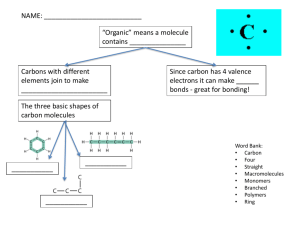
Human Biochemistry Amino Acids and Proteins • there are about 20 amino acids that occur naturally • they are the basic “building blocks” of life/proteins NH2CHRCOOH • condensation reactions will link amino acids together to form polypeptides that eventually fold up into proteins • enzymes are necessary! • water is formed and they link together with a peptide bond • peptide bonds YouTube (1:14) What is a protein video 3:38 Proteins have a complex structure which can be explained by defining four levels of structure Primary Structure • determined by the number, kind, and order of a.a. in the polypeptide • held together by simple peptide bonds Secondary Structure • the polypeptide then spontaneously folds into regular, repeating structure because of hydrogen bonding Tertiary Structure • highly specific looping and folding of the polypeptide because of the following interactions between their R-groups: – – – – covalent bonding hydrogen bonding ionic bonding London dispersion forces • this tertiary level is the final level of organization for proteins containing only a single polypeptide chain Quaternary Structure • linkage of two or more polypeptides to form a single protein in precise ratios and with a precise 3-D configuration. • Protein folding Quaternary Structure example Carbohydrates • most abundant class of biological molecules • range from simple sugars (glucose) to complex carbohydrates (starch) Monosaccharides • simplest sugars (single sugars) – • • all contain the empirical formula (CH2O) can be straight chains or cyclic form two common isomers of monosaccharides (C6H12O6) • • glucose fructose Disaccharides • two monosacharides bonded together by a condensation reaction that creates a glycosidic linkage • water is formed • three common disaccharides (don’t need to know this) 1. sucrose - common table sugar glucose + fructose 2. lactose - major sugar in milk glucose + galactose 3. maltose - product of starch digestion glucose + glucose Polysaccharides • starch- condensation of many glucose molecules • condensation of many glucose molecules to form long chains • serve principally as food storage and structural molecules in plants • three types of polysaccharides 1. Starches (plants) – serve as storage depots of glucose 2. Cellulose (plants) – most abundant polysaccharide on Earth – the major structural material of which plants are made (wood and plant fibers) – plant cell walls are among the strongest of biological structures 3. Glycogen – multi-branched that serves as a form of energy storage in animals and fungi Lipids 3 Main Types of Lipids • ‘lipid’ comes from lipos, the Greek word for fat • all are hydrophobic (water-fearing/insoluble in water) • greasy, oily 1. Triglycerides (fats and oils) • found in adipocyte cells that are in fatty tissue • a condensation reaction called ester linkage • types - saturated fat - do NOT contain C=C bonds - therefore straight chained and have high melting points - lard and butter - unsaturated fat - have double bonds between one (monounsaturated fats) or more (polyunsaturated fats) of the carbons in the chain - causes a kink in the carbon chain which prevents them from packing close together and therefore have low melting points (London dispersion forces are weaker) - vegetable oils Saturated vs. Unsaturated fatty acids (2:51) 2. Phospholipids • major structural components of cell membranes • polar “heads” love water (hydrophilic) • uncharged “tails” avoid water (hydrophobic) _ + + _ 3. Steroids • cholesterol is the most abundant and important steroid • lipoproteins – molecules made of proteins and fat – transport cholesterol around the body – low density lipoproteins (LDL) “bad cholesterol” • transport cholesterol to cells to be used • however, can build up and cause cardiovascular disease – high density lipoproteins (HDL) “good cholesterol” • doesn’t have much cholesterol, therefore, can absorb more cholesterol from the arteries and transports it back to the liver