Heat Study Guide: States of Matter, Energy Transfer
advertisement
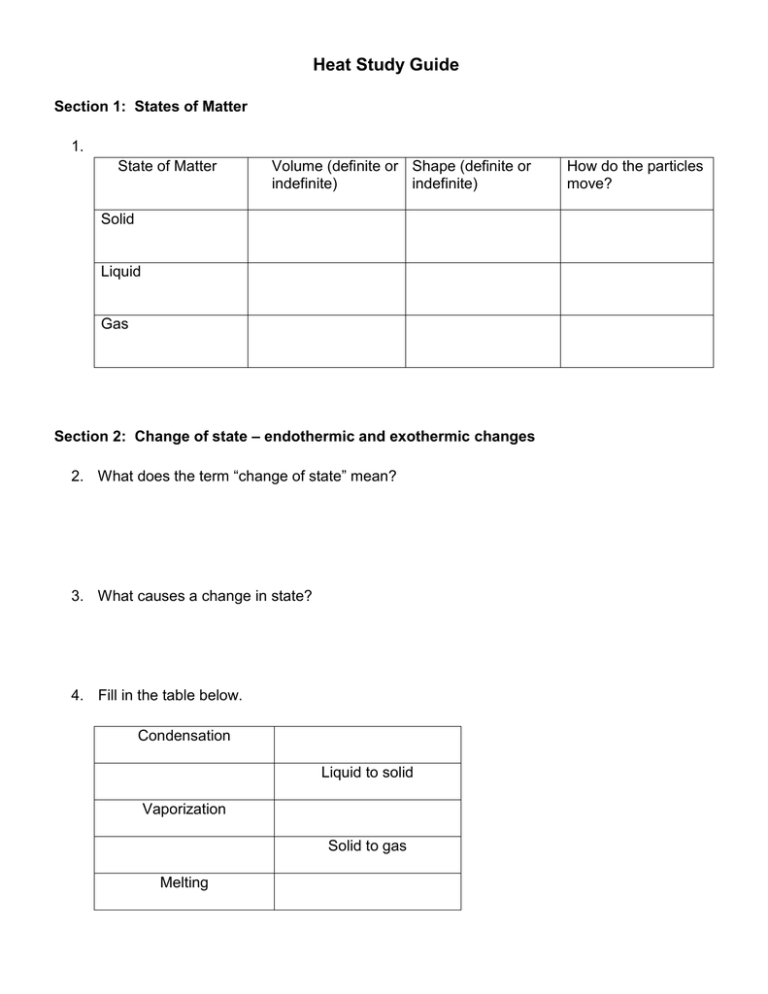
Heat Study Guide Section 1: States of Matter 1. State of Matter Volume (definite or Shape (definite or indefinite) indefinite) Solid Liquid Gas Section 2: Change of state – endothermic and exothermic changes 2. What does the term “change of state” mean? 3. What causes a change in state? 4. Fill in the table below. Condensation Liquid to solid Vaporization Solid to gas Melting How do the particles move? 5. Place the three states of matter (liquids, solids and gases) in the diagram below, according to their level of energy (least to greatest). Write in the name of each of the three endothermic changes of state in the correct place on the diagram.(melting, vaporization, sublimation) _____________ _____________ _____________ 6. When energy is taken in by a substance and changes state, it is called a(n) ______________________________________ change. 7. When energy is lost by a substance and it changes state, it is called a(n) ______________________________________ change Section 3 – Temperature and Thermal Energy 8. What is temperature? 9. What is kinetic energy? 10. How do we find an average? 11. How does the temperature of an object increase? 12. What are the three scales (languages) we can use when we talk about temperature? 13. The Celsius scale was developed based on the freezing point and boiling point of water. Fill in the table. Freezing pt. of water Boiling pt. of water Average human body temp. Celsius Fahrenheit 14. The Kelvin scale was developed with the idea that “zero” should mean no kinetic motion. What do we call this special number that means that none of the particles in a substance are moving at all? 15. What is thermal energy? 16. Which picture above has the highest temperature? Which picture has the most thermal energy? Why? Section 4 - Heat and Heat transfer 17. What is heat? 18. What is the difference between heat and temperature? 19. What is conduction? Give an example. 20. What is convection? Give an example. 21. What is radiation? Give an example. 22. Thermal energy moves from _____________objects/areas to _______________ objects/areas. 23. What is thermal expansion? Section 5 – Phase Change Diagrams 24. What does the term melting point mean? What does the term freezing point mean? 25. How are melting point and freezing point the same? 26. Is the melting point the same for all substances? Explain your answer. 27. What does the term boiling point mean? Use this graph to answer questions 28 to 35. 28. A point A, what state of matter in the matter in? 29. At point B, what is happening to the matter? 30. At point C, what begins to happen to the substance? What state or states of matter is the substance in, between points C and D? 31. Point D starts another change of state. What is it called? 32. What state or states of matter is the substance in between points D and E? 33. Right after point E on the graph, what state or states of matter is the substance in? 34. What is the melting point of this substance? What is the boiling point? How did you know? 35. Is there a change in temperature during a phase change (freezing, melting, etc)?