Unit 11 Energy, Changes of State, Solids and Liquids
advertisement

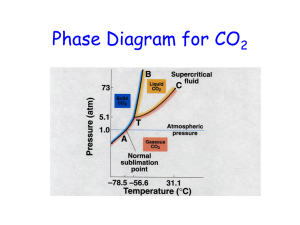
Unit 11 Energy, Changes of State, Solids and Liquids Thermodynamics – the study of energy Energy – the ability to do work or produce heat Units of energy: calorie (cal) – amount of energy required to rise the temperature of 1 gram of water 1 ˚C Joule (J) – SI unit – 1 calorie = 4.184 J Potential Energy – energy due to position or composition Kinetic Energy – energy due to the motion of the object and depends on mass (KE = ½ mv 2) Law of Conservation of Energy – energy can be converted from one form to another but can be neither created nor destroyed Work – force acting over a distance Temperature – measure of the random motion of the components of a substance (average KE) Heat – flow of energy due to a temperature difference Solar System – part of the universe on which we want to focus attention (reactants and products) Surroundings – everything else in the universe Calorimeter – device used to determine the heat associated with a chemical reaction Entropy (S) – measure of disorder and randomness The amount of energy required to change the temperature of a substance depends on: 1. Amount of substance (mass) 2. The amount of temperature change (ΔT) 3. Type or identity of the substance - each has a different specific heat (s) or (c) q = m ΔT s Specific heat capacity (s or c) – describes the amount of energy required to change the temperature of one gram of a substance by 1 ˚C, measured in J/g ˚C q (in joules) = energy to achieve the temperature change m (in grams) = mass of the substance ΔT (in ˚C) = change in temperature (Tfinal – Tinitial) Example 1: You have a 5.63 gram sample of solid gold and heat it from 21 ˚C to 32 ˚C. How much energy (in Joules) is required? The specific heat of gold is 0.13 J/g ˚C. q = m ΔT s = (5.63) (11) (0.13) = 8.0 J (round to 2 sig figs) Example 2: A 2.8 g sample of a pure metal required 10.1 Joules of energy to change its temperature from 21 ˚C to 36 ˚C. What is the metal’s specific heat capacity? What can we do now that we know it? q = m ΔT s s = 𝒒 𝒎 𝜟𝑻 = 𝟏𝟎.𝟏 (𝟐.𝟖)(𝟏𝟓) = 0.24 J/g ⁰C (round to 2 sig figs) Enthalpy (H) – heat for the reaction EXOTHERMIC -ΔH = energy released = exothermic reaction Energy transferred out of system by heat Products have less PE than reactants Energy shown as a product EXOTHERMIC Examples: Liquid water freezing to form ice Water vapor condensing into dew or raindrops Explosions Combustion ENDOTHERMIC +ΔH = energy absorbed = endothermic reaction Feels cold to touch (energy absorbed into system from surroundings) Products have more PE than reactants Energy shown as a reactant Examples: Ice cubes melting into liquid water Liquid water evaporating and becoming water vapor A cake baking in the oven Barium hydroxide and ammonium nitrate (a chemical cold pack) Review: Intermolecular forces: Intramolecular = bonds within a molecule = covalent Intermolecular = forces between molecules or ions 1. Ion-Ion Interactions Strongest: hold ions in a crystal structure 2. Hydrogen bonding Hydrogen bonded to highly electronegative ion (“FON”) 3. Dipole-dipole Interactions Polar molecules 4. London Dispersion Forces weakest Noble gases atoms and non-polar molecules “momentary’ dipoles in neighbor Heating/Cooling Curve: plot of temperature vs. time for a substance, where energy is added at a constant rate Molar Heat of Fusion (∆Hf) – the amount of heat necessary to melt one mole of a substance at its melting point (KJ/mol) q = ∆Hf (n) n = number of moles Example: 18 g of H2O is being melted at its melting point of 0 ˚C. How many kJ of energy are required to melt this ice? The ∆H f of water is 6.02 kJ/mol Use dimensional analysis because sometimes you will see 𝜟H in different units (like J/g) 18 g 1 mol 6.02 kJ = 6.0 kJ 18.02 g 1 mol Molar Heat of Vaporization (∆Hv) – the amount of heat necessary to boil (or vaporize) one mole of a substance at its boiling point q = ∆Hv (n) n = number of moles Example: 2.3 mole of H2O is being boiled at its boiling point of 100 ˚C. How many kJ are required to boil this water? The ∆Hv of water is 40.7 kJ/mol Don’t need molar mass because amount is already in moles 2.3 mol 40.7 kJ = 94 kJ 1 mol Phase Diagram: areas of stability of various phases in a chemical system at equilibrium Triple Point: all 3 phases exist in equilibrium Critical point: above this the gas will be a gas, cannot be liquified normal boiling point: boiling temperature under one atmosphere of pressure normal freezing point: the temperature in which a solid is formed under one atmosphere of pressure freezing point depression: add a solute to lower freezing temperature boiling point elevation: add a solute to raise boiling temperature vaporization: (evaporation) the change in state that occurs when a liquid evaporates to form a gas condensation: the process by which vapor molecules reform a liquid vapor pressure: the pressure of the vapor over a liquid at equilibrium in a closed container Changes of State Can you name the 5 states of matter? Describe (according to KMT) the states of matter: Solid: crystals – vibrate around fixed points, high IM forces Liquid: flow easily, lower IM forces, move more freely Gas: small volume (point masses); constant, rapid, random motion (extremely low IM forces); elastic (close to) collisions What is the name for each change of state? In the changes: melting vaporization sublimation Energy is added to the substance (absorbed) Moving from less → more freely (by overcoming IM forces) Increase temperature or decrease pressure In the changes: freezing condensation deposition Energy is given off to the surroundings Moving from more → less freely (allow IM forces to “mater” or “take over” Decrease temperature or increase pressure