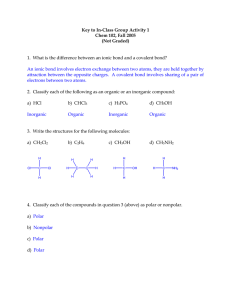
LAB: SHAPES OF COVALENT MOLECULES & POLARITY Introduction: The most common chemical bond between two atoms is a covalent bond. The covalent bond consists of a pair of shared electrons, one from each atom. If this pair of electrons is shared between two atoms of equal electro negativities, the bond would be called a nonpolar covalent bond. However, in most cases, the pair of electrons is shifted toward the more electronegative element. A partial negative charge results on one side of the bond and a partial positive charge on the other. This type of covalent bond is called polar covalent. Molecules composed of covalently bonded atoms may also be polar or nonpolar. For the molecule to be polar, it must, of course, have polar bonds. But the key factor for determining the polarity of a molecule is its shape. If the polar bonds (dipoles) are symmetrical around the central atom, they offset each other and the resulting molecule is nonpolar. However, if the dipoles are not symmetrical around the central atom, the electrons will be pulled to one end of this molecule and the resulting molecule is polar. Objectives: (1) Predict each molecule’s shape using your knowledge of hybridization. (2) Predict each molecule’s polarity on the basis of its shape. Equipment: Paper and pencil Procedure: 1. Prepare a data table according to the directions in the Analysis section below. 2. Complete your data table using the following compounds: 1. H2 5. SO3 9. CH4 13. CH3Cl 2. HBr 6. CO2 10. HClO 14. HCOOH 3. H2O 7. H2CO 11. O2 15. CO32- 4. NH3 8. C2H2 12. AlH3 16. NH4+ Analysis: Prepare a table for recording data for each of the 16 molecules. Include the following in your table: the formula, the Lewis dot structure, shared electron pairs, unshared electron pairs, total electron pairs, bonding orbitals, molecular shape, structural formula, and polarity. Use the table below as a guide: Formula HCl Lewis Shared Unshared Total Molecular Structural Structure e-Pairs e- Pairs e-Pairs Shape Formula H – Cl : 1 0 1 Linear H – Cl Polarity Polar Further Investigations: 1. On the basis of this experiment and your classwork, predict the a. type of bonding b. molecular shape c. molecular polarity for each of the following compounds (construct a table): 2. (1) HBr (3) BaCl2 (5) CI4 (2) SCl2 (4) NH3 (6) AlH3 Calculate the electronegativity difference and indicate the type of bond for the following attractions: (a) Na - Br (c) Se – O (e) Mg – Cl (b) 3. C-H (d) Br – Br What does the term isomer mean? (f) Al - I Key Formula Lewis Shared Unshared Total Molecular Structural Structure e-Pairs e- Pairs e-Pairs Shape Formula 1 0 or 3 1 or 4 Linear H – Cl Polar 1 0 or 3 1 or 4 Linear H – Br Polar 2 2 4 Bent HCl HBr H2O H Br Polarity Polar Trigonal NH3 3 1 4 Pyramid Polar al SO3 3 0 3 CO2 2 2 4 H2CO 3 0 3 C2H2 2 0 2 CH4 4 0 4 HClO 2 2 4 Trigonal Non Planar Polar Linear Polar Trigonal Planar Linear Polar Non Polar Tetra Non hedral Polar Bent Polar O2 AlH3 Ionic CH3Cl HCOOH Omit 2 2 4 Ionic Ionic Ionic 4 0 4 3 0 3 Linear Non O–O Polar Crystal Ionic Lattice Tetra Polar hedral Trigonal Polar Planar O CO32- 3 0 3 NH4+ 4 0 4 Trigonal Planar O C Non O Polar Tetra Non hedral Polar Further Investigation 1. On the basis of this experiment and your classwork, predict the a. type of bonding b. molecular shape c. molecular polarity for each of the following compounds (construct a table): (1) HBr (3) BaCl2 (5) CI4 (2) SCl2 (4) NH3 (6) AlH3 Compound Bond Molecular Polarity Type Shape HBr Covalent Linear Polar SCl2 Covalent Bent Polar 2. BaCl2 Ionic Crystal Lattice Ionic NH3 Covalent Trigonal Pyramidal Polar CI4 Covalent Tetrahedral Non-Polar AlH3 Ionic Crystal Lattice Ionic Calculate the electronegativity difference and indicate the type of bond for the following attractions: (a) Na - Br (c) Se – O (e) Mg – Cl (b) C-H Elements (d) Br – Br Electronegativity (f) Al - I Bond Type Difference 3. Na - Br Ionic C-H Covalent (Non-Polar) Se – O Covalent (Polar) Br – Br Covalent (Non-Polar) Mg – Cl Ionic Al - I Ionic What does the term isomer mean? Any of two or more compounds, radicals, or ions that contain the same number of atoms of the same elements but differ in structural arrangement and properties. Example