Name___________________ Period__________________ Chemistry Quiz
advertisement
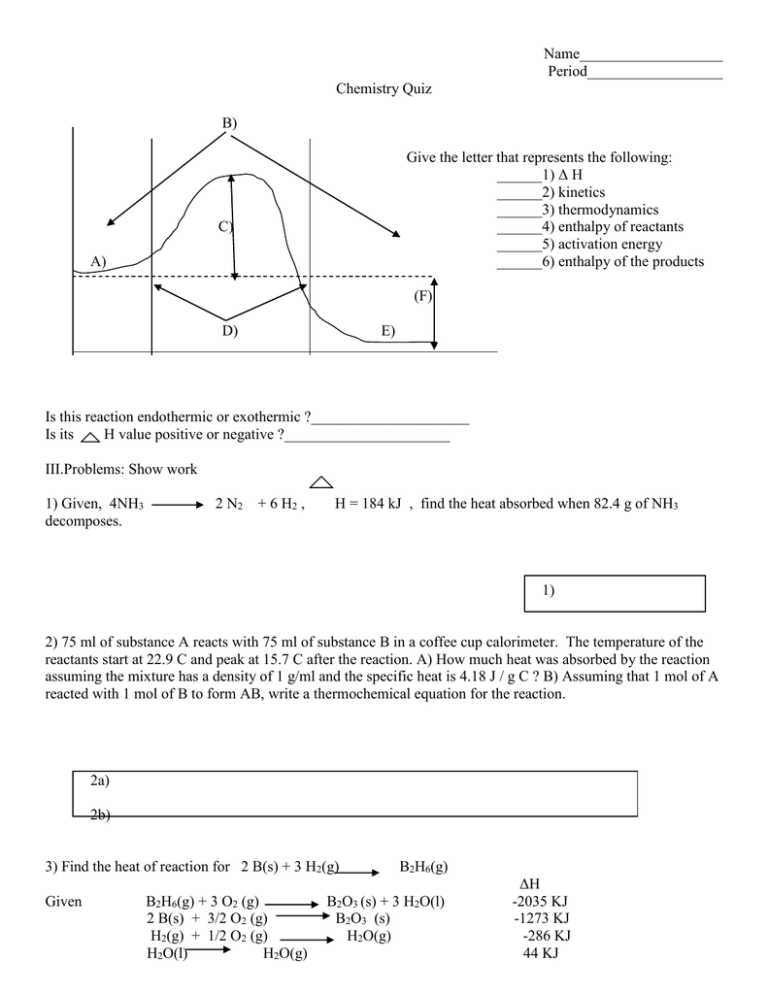
Name___________________ Period__________________ Chemistry Quiz B) Give the letter that represents the following: ______1) Δ H ______2) kinetics ______3) thermodynamics ______4) enthalpy of reactants ______5) activation energy ______6) enthalpy of the products C) A) (F) D) E) Is this reaction endothermic or exothermic ?_____________________ Is its H value positive or negative ?______________________ III.Problems: Show work 1) Given, 4NH3 decomposes. 2 N2 + 6 H2 , H = 184 kJ , find the heat absorbed when 82.4 g of NH3 1) 2) 75 ml of substance A reacts with 75 ml of substance B in a coffee cup calorimeter. The temperature of the reactants start at 22.9 C and peak at 15.7 C after the reaction. A) How much heat was absorbed by the reaction assuming the mixture has a density of 1 g/ml and the specific heat is 4.18 J / g C ? B) Assuming that 1 mol of A reacted with 1 mol of B to form AB, write a thermochemical equation for the reaction. 2a) 2b) 3) Find the heat of reaction for 2 B(s) + 3 H2(g) Given B2H6(g) + 3 O2 (g) 2 B(s) + 3/2 O2 (g) H2(g) + 1/2 O2 (g) H2O(l) H2O(g) B2H6(g) B2O3 (s) + 3 H2O(l) B2O3 (s) H2O(g) ΔH -2035 KJ -1273 KJ -286 KJ 44 KJ ` 3) 4) Use your thermodynamic tables to find Δ H , Δ G, and Δ S for the following reaction; 4 NH3 + 5 O2 4 NO + 6 H2O (all gases) ΔH ΔS ΔG Spontaneous? ____________ 5) Find use table. G @ 298 K for 4Fe + 3O2(g) 2Fe2O3(s) if Δ H is –1652 kJ and Δ S is –543 J/K. do not 5) Is this reaction spontaneous? 6) Predict the sign of Δ S for the following reactions. Negative or positive change? ______ ______ ______ ______ . N2(g) + 3 H2 (g) 3 O2(g) H2(g) 2 N2(g) + O2(g) 2 NH3(g) 2 O3(g) 2 H(g) 2N2O (g)