Determining the Formula of a Compound
advertisement
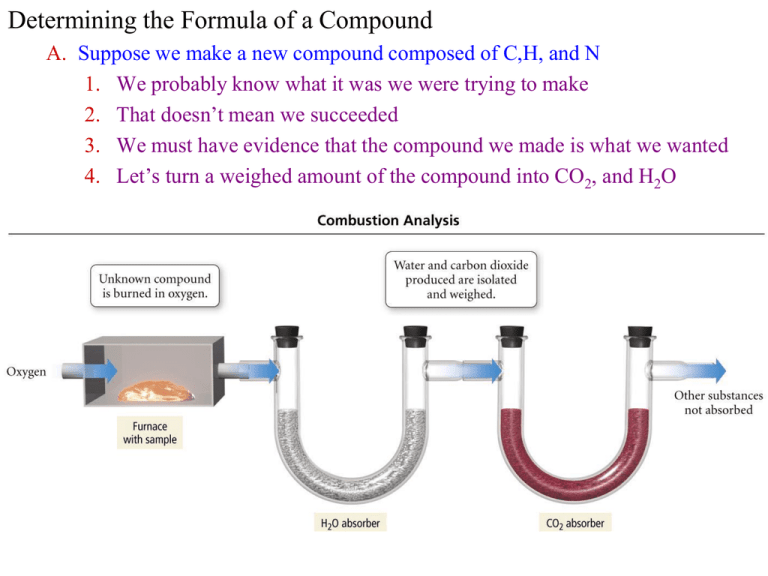
Determining the Formula of a Compound A. Suppose we make a new compound composed of C,H, and N 1. We probably know what it was we were trying to make 2. That doesn’t mean we succeeded 3. We must have evidence that the compound we made is what we wanted 4. Let’s turn a weighed amount of the compound into CO2, and H2O B. Analyzing the Results 1. 0.1156 g of our new compound gives 0.1638 g CO2 and 0.1676 g H2O a. (1C)(12.01g/C) + (2 O)(16.00g/O) = 44.01 g total for CO2 b. (2H)(1.008g/H) + (1 O)(16.00g/O) = 18.02 g total for H2O 2. Next, we find out how much carbon and hydrogen was in our sample 12.01 g C 44.01 g CO 2 0.1638 g CO 2 0.04470 g C 2.016 g H 0.1676 g H 2 O 0.01875 g H 18.02 g H O 2 3. Then, we determine the Mass Percents for our new compound 0.04470 g C 0.01875 g H 38.67% Carbon 0.1156 g total 0.1156 g total 16.22% Hy drogen 100% 16.22% 38.67% 45.11% Nitrogen 4. Finally, we need to determine the molecular formula of our compound a. The easiest way to do this is to work with a theoretical 100.00g b. We can then change the percent masses to grams of each element c. 38.67 g Carbon, 16.22 g Hydrogen, 45.11 g Nitrogen 5. Molecular formulas compare moles, not grams 1 mol C 3.220 mol Carbon 12.011 g C 38.67 g C 1 mol H 16.09 mol Hy drogen 1.008 g H 16.22 g H 1 mol N 3.219 mol Nitrogen 14.01 g N 45.11 g N a. Write the formula with these numbers of moles, then divide by the smallest number to get these subscripts as whole numbers C 3.220H16.09N 3.219 3.219 C1H 5 N1 b. This is called the Empirical Formula = smallest whole number ratio of elements in the formula c. Molecular Formula is the actual number of each element i. Could be C2H10N2 or C3H15N3 etc… ii. (C1H5N1)n all possible molecular formulas for this empirical formula 6. We can use a Mass Spectrometer on our compound to find its molecular mass is 31.06 g/mol. In this case CH5N is correct molecular formula. C. Sample Ex. 3.16—3.20 give more practice D. Hints for determining empirical and molecular formulas 1. Convert mass % to grams of the element in 100 total grams of sample Determine the Empirical Formula of an unknown if its Percent Composition is 47% Carbon, 47% Oxygen and 6.0% Hydrogen 100g 47gC 47gC 100g 100g 47gO 47gO 100g 100g 6.0gH 6.0gH 100g 2. Change these masses to moles by using the atomic mass of each element 1 mol C 47g C 3.9 mol C 12.01g 1 mol H 6.0 g H 6.0 mol H 1.008g 47 g O 1 mol O 2.9 mol O 16.00g 3. Divide each number of moles by the smallest to get a small # ratio C3.9 H 6 O 2.9 2.9 C1.34H 2.07O1 4. Round off to a whole # if molar #’s are close to that whole number 5. Multiply the whole ratio by a factor to get all numbers to whole numbers C1.34H2.07O1 C1.34H2 O1 x 3 C4 H6 O3 6. Multiply empirical formula by factor needed to give the correct molar mass a. Molar Mass of unknown = 204.2 g/mol but C4H6O3 = 102.09 g/mol b. Correct Molecular Formula: C4H6O3 x 2 = C8H12O6 III. Chemical Reactions A. Chemical Reaction Basics 1. Reactions involve chemical changes in matter resulting in new substances 2. Reactions rearrange and exchange atoms to produce new molecules 3. Elements are not transmuted during a reaction CH4 Reactants Products + O2 -------> CO2 + H2O B. Evidence of Chemical Reactions 1. visual clues (permanent): color change, precipitate formation, gas bubbles, flames, heat release, cooling, light 2. other clues: new odor, permanent new state IV. Chemical Equations A. Describing Chemical Reactions 1. Chemical Equation is a shorthand way of describing a reaction 2. Provides information about the reaction a. States and Formulas of reactants and products b. Relative numbers of reactant and product molecules that are required c. Weights of reactants used and of products that can be made B. Conservation of Mass 1. Matter cannot be created or destroyed 2. In a reaction, all the atoms present at the beginning are present at the end 3. The mass of the reactants will be the same as the mass of the products C. Combustion of Methane: Balancing a simple chemical equation 1. Methane gas burns to produce carbon dioxide gas and liquid water a. CH4(g) + O2(g) CO2(g) + H2O(l) b. This equation is Not Balanced 1C+4H + 2O 1C+2O +2H+O 1C+2H+3O 2. To obey conservation of mass, we must balance the equation a. We need equal numbers of each element on each side of the equation b. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) 1C + 4H + 4O 1C + 4H + 4O c. We also represent what physical state each reactant/product is in D. Writing Equations 1. Use proper formulas for each reactant and product 2. Proper equation should be balanced a. obey Law of Conservation of Mass b. all elements on reactants side also on product side c. equal numbers of atoms of each element on reactant and product sides 3. Balanced equation shows the relationship between the relative numbers of molecules of reactants and products 4. can be used to determine mass relationships 5. symbols used after chemical formula to indicate state a. (g) = gas; (l) = liquid; (s) = solid b. (aq) = aqueous, dissolved in water V. Balancing Chemical Equations by Inspection A. Procedure 1. Count atoms of each element a. Polyatomic ion may be counted as one “element” if no change in the reaction Al + FeSO4 Al2(SO4)3 + Fe 1 SO4 3 b. if an element appears in more than one compound on the same side, count each separately and add CO + O2 CO2 1 + 2 O 2 2. Pick an element to balance 3. Find Least Common Multiple and factors to make both sides equal 4. Use factors as coefficients in equation if already a coefficient then multiply by new factor 5. Recount and Repeat until balanced B. When magnesium metal burns in air it produces a white, powdery compound magnesium oxide (burning means reacting with O2) 1. write the equation in words identify the state of each chemical magnesium(s) + oxygen(g) magnesium oxide(s) 2. write the equation in formulas a. identify diatomic elements (H2, O2, N2, F2, Cl2, Br2, I2) b. identify polyatomic ions c. determine formulas Mg(s) + O2(g) MgO(s) 3. Count the number of atoms on each side a. count polyatomic groups as one “element” if on both sides b. split count of element if in more than one compound on one side Mg(s) + O2(g) MgO(s) 1 Mg 1 2O1 4. Pick an element to balance avoid element in multiple compounds 5. Find least common multiple of both sides & multiply each side by factor so it equals LCM Mg(s) + O2(g) MgO(s) 1 Mg 1 1x2O1x2 6. use factors as coefficients in front of compound containing the element if coefficient already there, multiply them together Mg(s) + O2(g) 2 MgO(s) 1 Mg 1 1x2O1x2 7. 8. Recount Repeat Mg(s) + O2(g) 2 MgO(s) 1 Mg 2 2O2 2 Mg(s) + O2(g) 2 MgO(s) 2 x 1 Mg 2 2O2 C. Under appropriate conditions at 1000°C ammonia gas reacts with oxygen gas to produce gaseous nitrogen monoxide and gaseous water 1. Write the equation in words a. identify the state of each chemical b. ammonia(g) + oxygen(g) nitrogen monoxide(g) + water(g) 2. Write the equation in formulas NH3(g) + O2(g) NO(g) + H2O(g) 3. Count the number of atoms of each element on each side NH3(g) + O2(g) NO(g) + H2O(g) 1 N 1 3H2 2O1+1 4. pick an element to balance (avoid element in multiple compounds) 5. find LCM of both sides & multiply each side by factor so it equals LCM NH3(g) + O2(g) NO(g) + H2O(g) 1 N 1 2x3H2x3 2O1+1 6. use factors as coefficients in front of compound containing the element 2 NH3(g) + O2(g) NO(g) + 3 H2O(g) 1 N 1 2x3H2x3 2O1+1 7. Recount 2 NH3(g) + O2(g) NO(g) + 3 H2O(g) 2 N 1 6H6 2O1+3 8. Repeat 2 NH3(g) + O2(g) 2 NO(g) + 3 H2O(g) 2 N 1 x 2 6H6 2O1+3 9. Recount 2 NH3(g) + O2(g) 2 NO(g) + 3 H2O(g) 2 N 2 6H6 2O2+3 10. Repeat a. A trick of the trade, when you are forced to attack an element that is in 3 or more compounds – find where it is uncombined. You can find a factor to make it any amount you want, even if that factor is a fraction! b. We want to make the O on the left equal 5, therefore we will multiply it by 2.5 2 NH3(g) + 2.5 O2(g) 2 NO(g) + 3 H2O(g) 2 N 2 6H6 2.5 x 2 O 2 + 3 11. Multiply all the coefficients by a number to eliminate fractions (0.5 x 2, 0.33 x 3, 0.25 x 4, 0.67 x 3) 2 x [2 NH3(g) + 2.5 O2(g) 2 NO(g) + 3 H2O(g)] 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) 4 N 4 12 H 12 10 O 10 E. Ethanol (C2H5OH) is burned in air to produce carbon dioxide and water. F. When solid ammonium dichromate decomposes to produce solid chromium(III) oxide, nitrogen gas, and water vapor.









