Properties of Water
advertisement

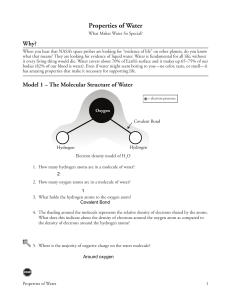
Properties of Water 1. 2. 3. 4. 5. Water is a molecule. Water consists of 2 atoms of hydrogen and one atom of oxygen. The hydrogen atoms are covalently bonded to the oxygen atom. Water exists in many forms, solid, liquid, gas. Water is a polar molecule because of the separation of charge that is a result of the oxygen's atom unequal sharing of electron in the covalent bonds with the hydrogen atoms. 6. The consequence is that one side of the water molecule has a slight positive charge and the other side has a slight negative charge. 7. These slight positive or negative charges are called partial charges, because of these partial charges water is able to hydrogen bond with other water molecules and other surfaces that exhibit a charged characteristic. 8. Water has a high surface tension. 9. Water has adhesive and cohesive properties. 10. Water has a high specific heat. 11. Water has high heats of fusion, sublimation, and vaporization. 12. Water has a high melting/freezing point. 13. Water has a high boiling point. 14. Water has a wide range of temperatures in which it can exist as a liquid. 15. Water is called the "universal solvent," indicating its ability to dissolve a wide variety of materials. 16. Solid water floats.