Honors Chemistry 1A Midterm Review Vocab
advertisement

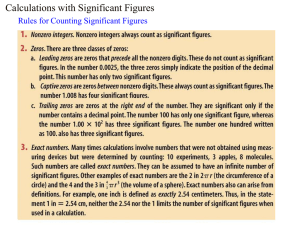
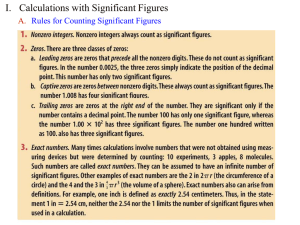
Honors Chemistry 1A Midterm Review Vocab scientific method solid heterogeneous mixture intensive property element liquid precision extensive property compound gas accuracy molecule Law of Conservation of mass homogenous mixture Law of multiple proportions Dalton’s Atomic Theory Know examples of the following: substances chemical changes heterogeneous mixtures physical property homogeneous mixtures chemical property physical changes 3) 0.234900 4) 0.00234900 Read to the proper number of sig figs 1) 2) How many sig figs? 1) 234.900 2) 234900 Solve and round to the proper number for sig figs. 1) (566.703 – 251.2) x 10.511 2) (876.31 x 2.451) + 8734.2389 Density 1. If an object has a density of 7.45g/cm3, find the density in kg/m3. 2. Calculate the density of an object with a mass of 134.2g that displaces 10.3mL of water. 3. Find the volume of an object with a mass of 329.8g and a density of 17,6g/cm3. 4. Determine the mass of an object with a volume of 66.4mL and a density of 4.36g/mL. Temperature Conversions 1) 35°C K 2) 378K °C Metric System Conversions (know prefixes from kilo- to nano-) 1) 1890mg kg 2) 4.37 x 10-4Dm mm 3) 4.25 x 10-3km3 cm3 Subatomic Particles Identify the following elements: 1) p+= 5 n0= 6 e-= 5 3) 55 X25 2) p+= 54 4) 55 n0= 77 X133 Tell number of protons, neutrons and electrons. 1) 55X25 p+= n0= e-= 2) What species is represented by the following information? 1) p+= 9 n0= 10 e-= 10 2) p+= 56 55 X133 Calculate the atomic mass of the following: 1) Li-6 6.015amu 7.42% Li-7 7.016amu 92.58% 23.9850amu 24.9858amu 25.9826amu p+= n0= 81 What is the empirical formula of each molecular formula? 1) C8H12O4 2) N2O4 2) Mg-24 Mg-25 Mg-26 e-= 54 78.70% 10.03% 11.17% *Know all of the groups of the periodic table *Know all of the charges produced when an element becomes an ion *Know the properties of metals *Be able to identify ionic and molecular compounds *Be able to write formulas for ionic, molecular and acidic compounds *Be able to write equations from words and balance the equation n0= e-= 54 e-=