Volume of a Irregular Solid
advertisement

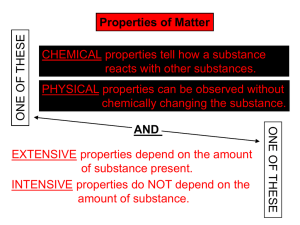
Density Calculations How do you calculate the density of a substance? Covered in This Lesson • This lesson will demonstrate how to calculate density to the correct number of significant figures(sig figs) using experimental measurements. – When calculating density we measure the mass and the volume of the sample. – The volume can be measured using one of several methods. Density Formual • When calculating density you measure the – mass of the sample using a balance – volume of the sample from one of the methods – apply the formula: mass/volume = density or m = V d Methods: Volume • There are three methods to measure the volume of a substance. The method used is determined by the type of substance. • Methods for the; 1. volume of liquid 2. volume of regular solid 3. volume of irregular solid. Volume of a Liquid • If the substance is a liquid, measure it directly in a graduated cylinder. Examples 1 • If a liquid sample has a mass of 4.83 g and a volume of 2.7 mL what is its density. 4.83 g = 1.788889 2.7 mL Because 2.7 has 2 sig figs the final answer is 1.8 g/mL Volume of a Regular Solid • If you have a regular solid like a cylinder, cube, or rectangular solid measure the dimensions needed and apply the volume formula. Examples 2 • If cubic solid sample has a mass of 40.53 g and the side is 2.55 cm, what is its density. V = s3 = (2.55 cm) 3 = 16.581375 cm3 D = 40.53 g = 2.44430875 16.581375 cm3 Because 2.55 has 3 sig figs the final answer is 2.44 g/cm3 Volume of a Irregular Solid • If you have an irregular solid use displacement, take initial volume of water in a graduated flask, submerging the sample in the flask, and take the final volume while the solid is submerged. Subtract final volume minus initial volume. Examples 3 • Find the density of a solid sample with a mass of 75.45 g and displacement is used to find the volume, the initial volume is 20.39 mL, the final volume is 73.57 mL. V = 73.57 mL – 20.39 mL = 53.18mL 75.45 g = 1.418766454 g/mL 53.18mL Since both mass and volume have 4 sig figs the final answer is 1.419 g/mL Practice Problems 1. Find the density of a liquid with a volume of 3.8 mL and a mass of 3.75 g. 2. Find the density of a rectangular solid with a length of 4.5 cm, a width of 2.5 cm, a height of 1.5 cm and a mass of 47.12 g. 3. Find the density of an irregular solid with a mass of 1.2 g, an initial volume of 25.7 mL and final volume of 18.4 mL when displacement is used. Answers Practice Problems 1. Density = 0.99 g/mL 2. Density = 2.79 g/cm3 3. Density = 8.8 g/mL