Osmosis in Plant Cells
advertisement

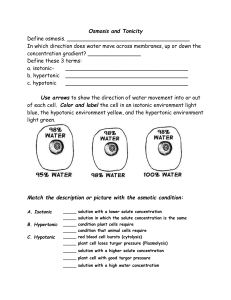
Osmosis in Plant Cells Plasmolysis • Observe Elodea leaves via a wet mount of the sample •Note location of central vacuole and chloroplasts in each sample Hypertonic Hypotonic Red blood cells in hypertonic solution Crenate (spiky, not smooth edge) RBC in isotonic solution Plump round cells HYPERTONIC • A Hypertonic solution contains a higher concentration of electrolytes than that found in body cells. If such a solution is allowed to enter the blood stream, the osmotic pressure difference between the blood and the cells will cause water to flow out of the cells, which will then shrink. This may cause serious harm, or even be fatal. • Consequently, it is essential when blood transfusions are given, or blood replacement products are used, that the electrolyte concentration in the material to be given to a patient matches that of the body. HYPOTONIC • Having a lesser osmotic pressure in a fluid compared to another fluid, as in a ‘hypotonic solution ISOTONIC • A solution that has the same salt concentration as the normal cells of the body and the blood. PLASMOLYSIS • Plasmolysis is the process in plant cells where the plasma membrane pulls away from the cell wall due to the loss of water through osmosis.