Osmosis in Elodea Cells Worksheet
advertisement

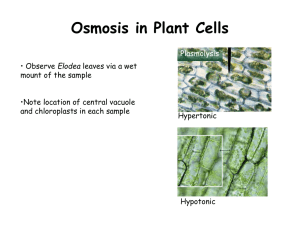
Name: Period: Osmosis in Elodea Cells Objective: In order to understand the effect of osmosis in a typical cell, we will view three animations showing typical elodea (a freshwater plant) cells in isotonic, hypertonic, and hypotonic situations. Before we begin: In the situation of saltwater, the salt is considered the _________________ while the water is considered the _________________. The combination of the two creates saltwater, which is considered a _______________________. Follow the directions below to get examples of cell reactions when placed in different types of solutions. 1. Visit the Anatomy and Physiology videos site at: http://www.linkpublishing.com/videotransport.htm 2. Scroll down to the Elodea – Osmosis section. 3. Using your notes, describe an isotonic solution: 4. Click on “WATCH VIDEO OF FRESH (NORMAL) ELODEA”. 5. Watch the animation and sketch a set of cells in this isotonic solution. 6. Return to the main page. 7. Using your notes, describe a hypertonic solution: 8. Click on “WATCH VIDEO OF NORMAL ELODEA SUBJECTED TO A HYPERTONIC SOLUTION”. 9. Watch the animation and sketch a set of cells in this hypertonic solution. Ensure that your sketch shows the difference between these cells and those in an isotonic solution! 10. Return to the main page. 11. Using your notes, describe a hypotonic solution: 12. Click on “WATCH VIDEO OF PLASMOLYZED ELODEA SUBJECTED TO A HYPOTONIC SOLUTION” (this animation shows what happens when the hypertonic cells are now placed in a hypotonic solution!) 13. Watch the animation and sketch a set of cells in this hypotonic solution. Ensure that your sketch shows the difference between these cells and those in an isotonic or hypertonic solution! Conclusions: Answer the following questions using the concepts of osmosis and diffusion in your answers! 1. What is the shape of the typical Elodea cell? 2. Using the sketches for one cell, draw the progression of a freshwater elodea cell being placed in a hypertonic solution and then in a hypotonic solution. 3. Why do grocery store owners spray fresh fruits and vegetables with water? 4. Roads are sometimes salted to melt ice. What does this do to plants around the roadside and why? 5. If a saltwater cell is placed in freshwater it may swell and pop. An elodea cell, however, will not. Explain why this is the case. 6. If a bowl of fresh strawberries is sprinkled with sugar, a few minutes later the berries will be covered with juice. Why?