Chemistry 11 Chemical Bonding Electronegativity – the tendency of an atom
Chemistry 11
Chemical Bonding
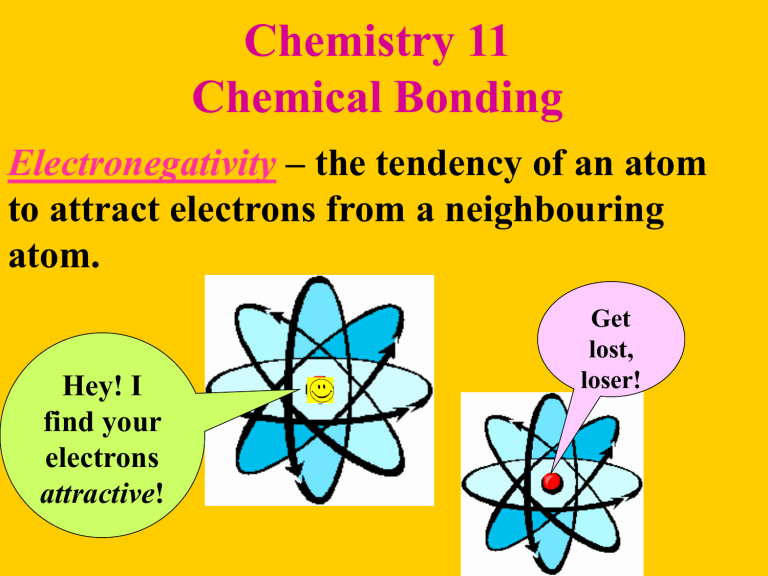
Electronegativity
– the tendency of an atom to attract electrons from a neighbouring atom.
Get lost, loser!
Hey! I find your electrons
attractive!
Electronegativity increases as you move from left to right.
Valence Electrons
– electrons in the outermost occupied energy level. (s and p electrons outside the core)
Valence electrons can be represented by
“dots” drawn around the atom.
Gilbert Newton Lewis
Invented “Electron-dot” formulas or “Lewis
Structures”
I’m so tired of writing all those useless inner electrons, in the
Bohring models!
When the electronegativities of two atoms are quite
different
from each other:
One atom loses an electron (or electrons)
The other atom gains an electron (or electrons)
This results in an
Ionic Bond
.
Chemical Bonding (Choose Ionic Bonds) crystal lattice viewer
NaCl
Crystal Lattice
Li
A Li Atom
+
Li
A Li + Ion
F
An F Atom
-
F
An F Ion
Be
F
An F Atom
F
An F Ion
-
A Be Atom
Be
2+
A Be 2+ Ion
F
An F Atom
-
F
An F Ion
The
melting points
of some Ionic
Compounds are as follows:
NaF 993 o C
KCl 770 o C
LiCl 605 o C
These high melting points are experimental evidence that Ionic Bonds are VERY STRONG . (Hard to break just by heating).
When Electronegativities of bonding atoms are the same (as they are in diatomic molecules) or close to the same, they
SHARE electrons.
Bonds formed when atoms share electrons are called
Covalent Bonds
.
In diatomic molecules (like H
2 or Cl
2 electronegativities of both atoms are
), the exactly the same so electrons are shared equally!
Covalent Bond animation
In
Covalent
bonds, electrons are
Shared
H H
Covalent bonds in large networks
(Network Bonding) gives rise to substances with very high melting points.
diamond structure
Diamonds are “forever”!
Some melting points of
Network Solids
:
Diamond (Carbon) 3550 o C
Silicon Carbide (SiC) 2700 o C
Boron Nitride (BN) 3000 o C
Covalent bonds are very strong !
When electrons are shared unequally between two atoms, the bond is called Polar Covalent . A type of
PC bond formed when “H” from one atom attracts “O” or “N” from another atom is called
Hydrogen Bonding . polar covalent bonds
Hydrogen Bonding in Water gives rise to the structure of ice when water solidifies.
Hydrogen bonds between the
“bases” hold the two strands of
DNA together.
Bonds
within
molecules that hold the atoms of a molecule together are called
intramolecular bonds
. They are strong covalent bonds.
Covalent Bonds
I
I
I
I
I
I
I
I
The covalent
intramolecular bond in I
2 is very strong.
I
I
I
I
There are weaker
intermolecular forces which hold covalent molecules together in a molecular solid.
A
dipole
is a partial separation of charge which exists when one end of a molecule has a slight positive charge and the other end has a slight negative charge. Eg. A water molecule has two dipoles.
The Greek letter
“delta” means
“partial” d
Just by pure chance, there are some times when both electrons in helium are on the same side. This forms temporary dipoles e e -
+2 e e -
+2
He He d d + d d +
The weak attractive forces between the
(+) side of one molecule and the (-) side of another molecule are called London
Forces
I
I
I
I
I
I
I
I
The covalent
intramolecular bond in I
2 is very strong.
I
I
I
I
There are weaker
intermolecular forces which hold covalent molecules together in a molecular solid.
These are called London
Forces . Since they are relatively weak, Iodine has a low melting point.
Lewis Structures (Electron-dot formulas) for Ionic Compounds.
Remember, in an ionic compound, the metal loses e ’s and the non-metal gains.
There is no sharing. Here is the e-dot formula for sodium chloride (NaCl)
Na +
Cl
Here is the e-dot formula (Lewis Structure) for the ionic compound MgF
2
:
F Mg 2+
F
Notice, there is no sharing. The F atoms took both valence e ’s from Mg, forming ions which do not share electrons. The + and – charges on the ions cause them to attract each other.
Electron-dot Formulas (Lewis Structures) for Covalent Compounds .
When atoms form covalent bonds, they are trying to achieve stable noble gas electron arrangements:
Hydrogen will share e ’s until it feels 2 e ’s like Helium.
Other elements share e ’s to achieve what is called a “
Stable Octet
” (8 valence e ’s)
Electron-dot formula for Methane (CH
4
)
H
H
Here is a Carbon atom (4 val e ’s) and four Hydrogen atoms (1 val e each)
C
H
H
Electron-dot formula for Methane (CH
4
)
Now they have formed a stable molecule. Each
C atom “feels” like it has a stable octet.
H
H
C
H
H
Each H atom
“feels” like a stable “He” atom with 2e s
Electron-dot formula for Ammonia (NH
3
)
H
N
Here is a Nitrogen atom (5 val e ’s) and three Hydrogen atoms (1 val e each)
H
H
Electron-dot formula for Ammonia (NH
3
)
“N” now feels like it has a stable octet
H N
H
H
Each “H” feels like it has 2 e like
Helium.
Write the electron-dot formula for CF
4
Because “F” is a halogen, it has 7 valence e s, so you must show all 7 red dots around each
“F” atom!
F
F
C
F
F
Write the electron-dot formula for H
2
S
S H
H
The two H’s
MUST be at right angles to each other!!
Write the Electron-Dot Formula for SeF
2
Se
F
F
Because “F” is in
Group 17, they have
7 valence e s, so they must have 7 red dots around them.





