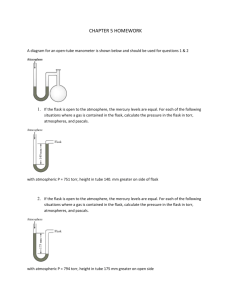
Jan 16 Gas Laws and More !!!!
advertisement

Jan 16 Gas Laws and More !!!! 1. The lid is tightly sealed on a rigid flask containing 4.20 L O2 at 27 °C and 0.969 atm. If the flask is heated to 81 °C, what is the pressure in the flask? 2. A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L. What will the gas pressure be if the volume is increased to 321 L at 125°C? 3. A balloon is filled with N2 gas to a volume of 1.92 L at 37 °C. The balloon is placed in liquid nitrogen until its temperature reaches -142 °C. Assuming the pressure remains constant, what is the volume of the cooled balloon? 4. A sample of nitrogen gas is confined to a 14.0 L container at 375 torr and 37.0°C. How many grams of nitrogen gas are in the container? 5. What volume of Ar at 35 °C and 1.75 atm contains the same number of particles as 0.655 L of Cl2 at 15 °C and 3.00 atm? 6. A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm. What is the molar mass of the gas? 7. A 48.0 L gas cylinder contains 498 g H2 at 25 °C. What is the pressure inside the cylinder? 8. If the density of N2 gas in a flask is 0.243 g/L at 18 °C, what is the pressure of nitrogen in the flask if it is heated to 36 °C? 9. Propane, C3H8, reacts with excess oxygen to produce carbon dioxide gas and water. What volume of CO2, measured at 27.2 °C and 0.918 atm is produced from the reaction of 10.1 g C3H8 with excess oxygen? Hint: Write a balanced equation. 10. Magnesium metal (0.100 mol) and a volume of aqueous hydrochloric acid that contains 0.500 mol of HCl are combined and react to completion. How many liters of hydrogen gas, measured at STP, are produced? Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g) 11. Ammonia gas is synthesized according to N2(g) + 3 H2(g) → 2 NH3(g). If 1.56 L N2 react with 4.32 L H2, what is the theoretical yield (in liters) of NH3? Assume that the volumes of reactants and products are measured at the same temperature and pressure. 12. Calculate the root-mean-square speed of methane, CH4 (g), at 78°C. 14. If a gas effuses 2.165 times faster than Xe, what is its molar mass? 15. (a) A sample of hydrogen gas was collected in a large gas buret. The pressure in the buret was measured as 764.2 torr, the temperature was 23.0 °C, and the buret contained 511 mL of the collected gas. How many moles of hydrogen were collected? (b) A sample of hydrogen gas was collected by displacement of water in a large gas buret. The total pressure in the buret was measured as 764.2 torr, the temperature was 23.0 °C, and the buret contained 511 mL of the collected gas. How many moles of hydrogen were collected? (At 23.0 °C, the vapor pressure of water is 21.1 torr.) 16. A gas mixture contains 5.00 liters of a mixture of nitrogen gas and oxygen gas. The total pressure in the tank is 38.6 atm when the temperature is 25.0 °C, and the mole fraction of oxygen is 0.400. How much does the oxygen in the tank weigh?