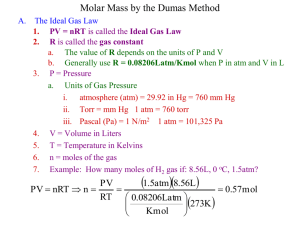
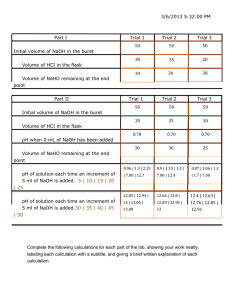
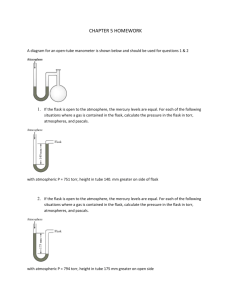
Chem 162 Ready for class? Gases
advertisement
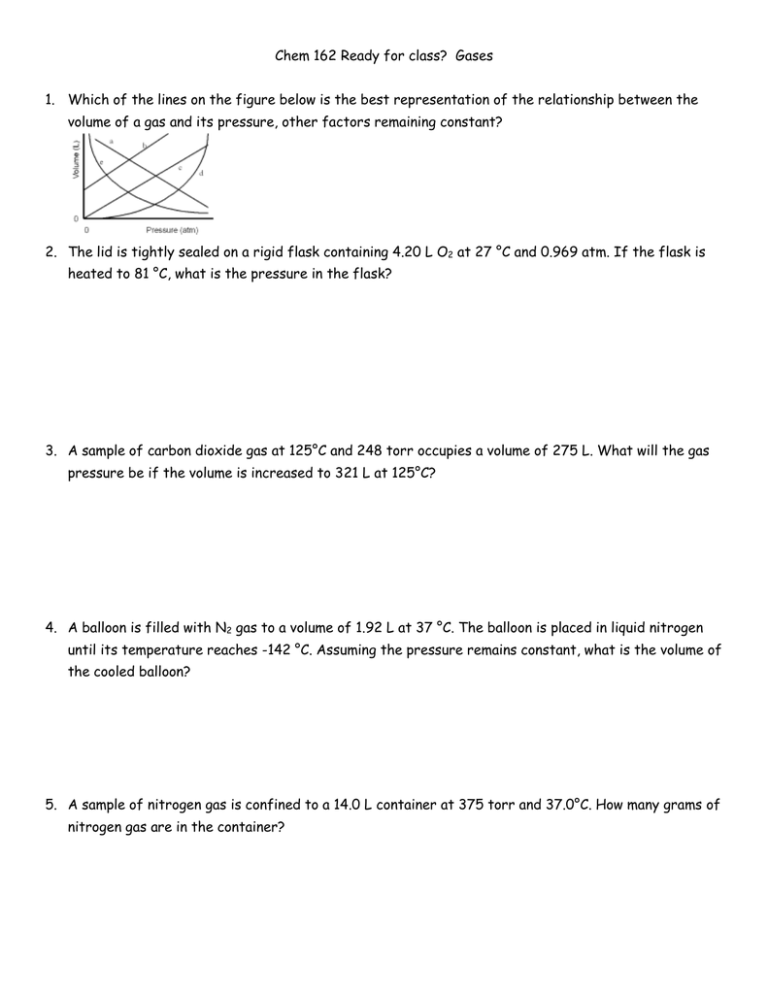
Chem 162 Ready for class? Gases 1. Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its pressure, other factors remaining constant? 2. The lid is tightly sealed on a rigid flask containing 4.20 L O2 at 27 °C and 0.969 atm. If the flask is heated to 81 °C, what is the pressure in the flask? 3. A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L. What will the gas pressure be if the volume is increased to 321 L at 125°C? 4. A balloon is filled with N2 gas to a volume of 1.92 L at 37 °C. The balloon is placed in liquid nitrogen until its temperature reaches -142 °C. Assuming the pressure remains constant, what is the volume of the cooled balloon? 5. A sample of nitrogen gas is confined to a 14.0 L container at 375 torr and 37.0°C. How many grams of nitrogen gas are in the container? 6. What volume of Ar at 35 °C and 1.75 atm contains the same number of particles as 0.655 L of Cl2 at 15 °C and 3.00 atm? 7. A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm. What is the molar mass of the gas? 8. A 48.0 L gas cylinder contains 498 g H2 at 25 °C. What is the pressure inside the cylinder? 9. If the density of N2 gas in a flask is 0.243 g/L at 18 °C, what is the pressure of nitrogen in the flask if it is heated to 36 °C? 10. Propane, C3H8, reacts with excess oxygen to produce carbon dioxide gas and water. What volume of CO2, measured at 27.2 °C and 0.918 atm is produced from the reaction of 10.1 g C3H8 with excess oxygen? Hint: Write a balanced equation. 11. Magnesium metal (0.100 mol) and a volume of aqueous hydrochloric acid that contains 0.500 mol of HCl are combined and react to completion. How many liters of hydrogen gas, measured at STP, are produced? Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g) 12. Ammonia gas is synthesized according to N2(g) + 3 H2(g) → 2 NH3(g). If 1.56 L N2 react with 4.32 L H2, what is the theoretical yield (in liters) of NH3? Assume that the volumes of reactants and products are measured at the same temperature and pressure. 13. Calculate the root-mean-square speed of methane, CH4 (g), at 78°C. Root mean square speed = u = [3RT/(molar mass) ] ^ (1/2) Think about which R you are going to use and make sure to show the units! 14. If a gas effuses 2.165 times faster than Xe, what is its molar mass? 15. (a) A sample of hydrogen gas was collected in a large gas buret. The pressure in the buret was measured as 764.2 torr, the temperature was 23.0 °C, and the buret contained 511 mL of the collected gas. How many moles of hydrogen were collected? (b) A sample of hydrogen gas was collected by displacement of water in a large gas buret. The total pressure in the buret was measured as 764.2 torr, the temperature was 23.0 °C, and the buret contained 511 mL of the collected gas. How many moles of hydrogen were collected? (At 23.0 °C, the vapor pressure of water is 21.1 torr.) 16. A gas mixture contains 5.00 liters of a mixture of nitrogen gas and oxygen gas. The total pressure in the tank is 38.6 atm when the temperature is 25.0 °C, and the mole fraction of oxygen is 0.400. How much does the oxygen in the tank weigh? 17. The van der Waals equation of state for a real gas is n 2 a V nb P nRT V 2 In this equation, the van der Waals constant, a, represents a correction for _______ and b represents a correction for _______________________.