Titration Prelab
advertisement

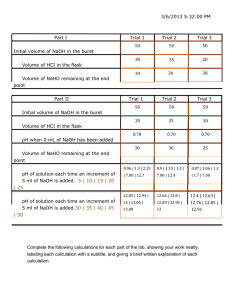
Acid- Base Prelab Define the following terms: 1pt each 1) Titration 2) Equivalence point 3) End point 4) Buret Determine the molarity of a 15.0 mL sample of nitric acid require 13.63 mL of .075 M sodium hydroxide to reach a phenolphthalein end point. (2pts) a) Write the neutralization equation b) Determine the molarity-show your calculations! Rationalize the following errors, that is tell me whether the observed results would be higher, lower, or the same as the true value. All situations refer to the above titration! (4pts) a) b) c) d) An air bubble was in the buret when the titrant was added the flask. Water was added the flask with the acid to give a larger volume of solution. Unknown to you some residual soap was in the flask that contained the acid. When the stock solution of sodium hydroxide was prepared slightly more water was added than should have been.