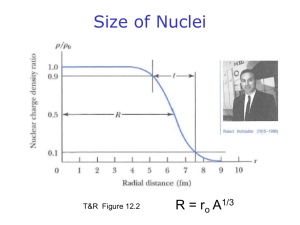
NUCLEAR CHEMISTRY: INTRO
advertisement
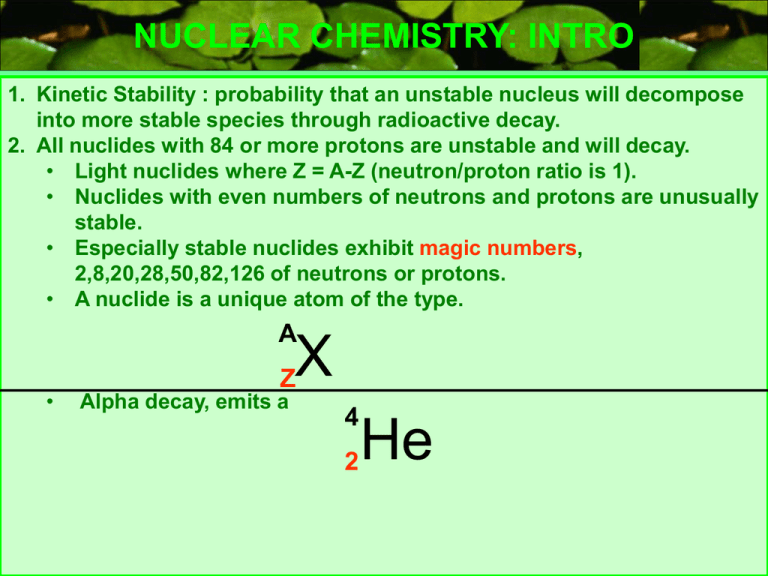
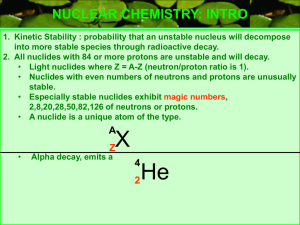
NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with even numbers of neutrons and protons are unusually stable. • Especially stable nuclides exhibit magic numbers, 2,8,20,28,50,82,126 of neutrons or protons. • A nuclide is a unique atom of the type. A X • Z Alpha decay, emits a 4 2 He NUCLEAR CHEMISTRY: STABILITY GRAPH #n 120 110 100 90 80 70 60 50 40 30 20 10 20 p 40 p 60 p 80 p 100 p NUCLEAR CHEMISTRY: BETA DECAY 14 6 1. 2. 3. 4. 5. C 14 N 7 0 + e BETA PARTICLE -1 BETA DECAY THE ATOMIC NUMBER OF THE PRODUCT INCREASES. NUCLIDES ABOVE THE PENNINSULA (ZONE) OF STABILITY DECAY WITH BETA DECAY(SEE GRAPH ON OTHER SLIDE). PENETRATING RADIATION. THE BETA PARTICLE CONES FROM THE DECOMPOSITION OF A NEUTRON TO A PROTON AND BETA PARTICLE. THE BETA PARTICLE IS AN ELECTRON “BORN” IN THE NUCLEUS. BETA DECAY IS SPONTANEOUS, NOTICE ONLY ONE REACTANT. 1 n 0 1 p 1 0 + -1 e NUCLEAR CHEMISTRY: ALPHA DECAY 238 U 92 4 He 2 234 + Th 90 ALPHA PARTICLE 1. 2. 3. 4. 5. 6. 7. ALPHA DECAY THE ATOMIC NUMBER OF THE PRODUCT DECREASES BY 2. THE MASS NUMBER OF THE PRODUCT DECREASES BY 4. ALPHA RADIATION IS NON PENETRATING TO HUMAN SKIN, HOWEVER IT CAN BE INGESTED. COMMON MODE OF DECAY FOR HEAVY RADIOACTIVE NUCLIDES. NEUTRON/PROTON RATIO INCREASES. THE ALPHA PARTICLES ARE POSITIVE, THESE HIGH ENERGY He ATOMS HAVE LOST THE ELECTRONS, ARE REPELLED BY POSITIVE ELECTRODES AND ARE AFFECTED BY MAGNETIC FIELDS. ALPHA DECAY IS SPONTANEOUS. NUCLEAR CHEMISTRY: POSITRON EMISSION 22 11 Na 0 e +1 22 + Ne 10 POSITRON 1. 2. 3. 4. POSITRON EMISSION DECAY MODE FOR NUCLIDES BELOW ZONE OF STABILITY. CHANGES A PROTON TO A NEUTRON. PRODUCT HAS A HIGHER NEUTRON TO PROTON RATIO. THE POSITRON IS THE ANTIPARTICLE TO AN ELECTRON THE REACTION OF A POSITRON WITH A BETA PARTICLE PRODUCES GAMMA RADIATION . 0 e +1 0 + e -1 0 GAMMA 0 NUCLEAR CHEMISTRY: ELECTRON CAPTURE 201 80 Hg 0 + e -1 201 Au 79 + 0 GAMMA 0 INNER ORBITAL SHELL ELECTRON ELECTRON CAPTURE 1. AN INNER SHELL ELECTRON IS CAPTURED BY THE NUCLEUS. 0 e +1 0 + e -1 0 GAMMA 0 NUCLEAR CHEMISTRY: ELECTRON CAPTURE ln (N0/N) = kt Log N0 N ( ) t1/2 =0.693/k E = c2 m = kt 2.303 Example problem, binding energy OBJECTIVE calculate the binding energy per nucleon of E = c2 m Mass defect equation N –14, nuclear mass is 13.999234 mass of proton mass of neutron mass nucleus m = (7(1.0072765) + 7(1.0086649)) – 13.999234 = 0.112536 amu E = 0.112536 amu Avagodro’s # 1g 6.0221 * 1023 amu x 1kg 1000g x 8.987551*1016 m2 s2 = 1.67682 * 10-11 kg m2/s2 = 1.67682 * 10-11 J For 14N, A=14 Binding energy/nucleon = 1.67682 * 10-11 J/ 14 = 1.19773 * 10-12 J/nucleon Example problems:Half Life Calculate the mass of Co-60 that remains from a 0.0100 g sample after 1.00 year has elapsed. k= 0.693 = 0.693 = 0.132y t1/2 5.27/y 1-FIRST FIND k FROM HALF LIFE. Co-60 Half life,from tables 2-FIND N0/N RATIO Log N0 N ( ) = kt 2.30 Log N0 N = (0.132/y)(1.00y) = 0.0570, ANTILOG OF 0.0570 IS 1.14 2.303 ( ) N0 = 1.14 = 0.0100g N N ; N = 0.00877g OF 60 C 27 AFTER 1 YEAR