2 KOH (s) + H SO (g) K
advertisement
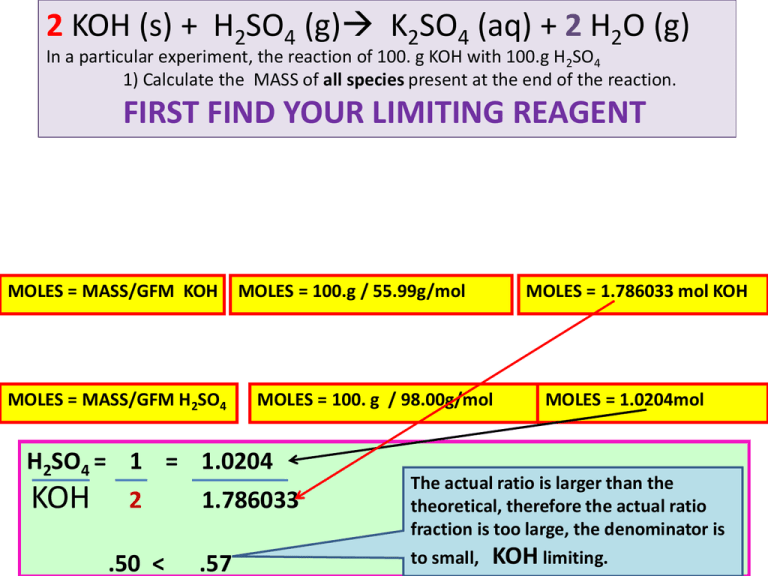
2 KOH (s) + H2SO4 (g) K2SO4 (aq) + 2 H2O (g) In a particular experiment, the reaction of 100. g KOH with 100.g H2SO4 • 1) Calculate the MASS of all species present at the end of the reaction. FIRST FIND YOUR LIMITING REAGENT MOLES = MASS/GFM KOH MOLES = MASS/GFM H2SO4 MOLES = 100.g / 55.99g/mol MOLES = 100. g / 98.00g/mol H2SO4 = 1 = 1.0204 KOH 2 .50 < MOLES = 1.786033 mol KOH MOLES = 1.0204mol 1.786033 The actual ratio is larger than the theoretical, therefore the actual ratio fraction is too large, the denominator is .57 to small, KOH limiting. ICE CHART SOLUTION SKILL THE ICE CHART WILL ALLOW YOU TO CALCULATE MOLES OF ALL SPECIES AFTER THE REACTION IS COMPLETED. MOLES OF ALL PRODUCTS. MOLES OF ALL EXCESS REACTANTS REMAINNING. THE FASTEST WAY TO APPROACH A REACTION IN WHICH YOU ARE CALCULATING MANY SUBSTANCES AT ONCE. 2KOH(s) + H2SO4(g) K2SO4(aq) +2H2O(g) I 1.786033 mol C -2X 1.0204mol E 1.0204 –X 0.0 -X 0.0 0.0 +X +2X +X +2X DETERMINING THE VALUE OF “X” 1. X IS DETERMINED BY THE LIMITING REAGENT. 2. IN THIS PROBLEM KOH, 1.786033 mol = 2X FROM THE LIMITING REAGENT KOH. X = 0.893 MOL. Quantities of all species are calculated from x: 1. THE EXCESS REAGENT IS SULFURIC ACID, WHICH IS 1.0204 –X MOLES = MASS/GFM K2SO4 1.0204 – 0.893 0.893mol= MASS/173.99 g 0.1274 MOLE OF H2SO4 REMAINS MASS = 153.87 g K2SO4 154. g 2. K2SO4 PRODUCED IS X, 0.893 MOLE 3 WATER IS 2X, 2(0.893) = 1.786 MOLE. MOLES H2O= MASS/GFM H2O 1.786 MOLE = MASS/17.99 : MASS = 32.103g = 32.1g





