PPT Nature of Electrons
advertisement
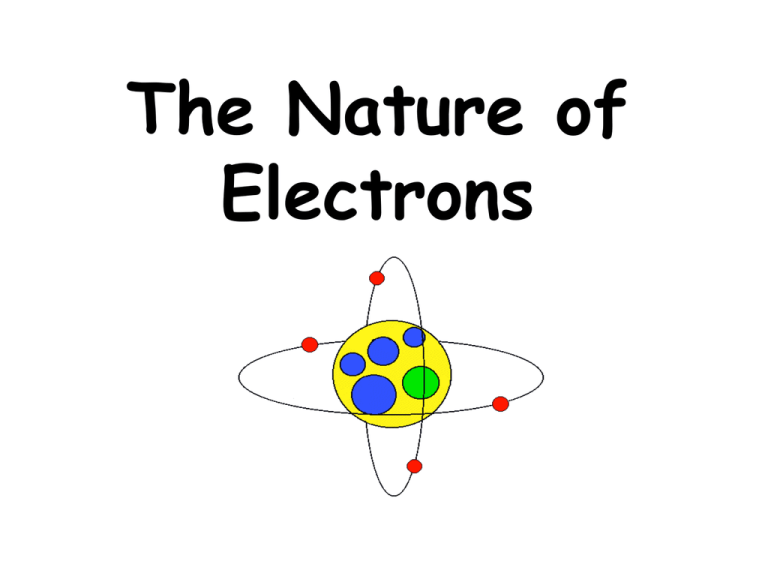
The Nature of Electrons • Scientists noticed when certain elements are placed in a flame, they emit light! • The light given off when an element is heated is called it’s atomic emission spectrum • The atomic emission spectrum is a different color for every element! • The color is like the “signature” of that element • It can be used to identify the element like a fingerprint can identify you! No two elements have the same atomic emission spectrum!!! Scientists began to wonder . . . • Rutherford’s model is good, but it doesn’t explain some things Rutherford’s model didn’t explain: 1. why different elements react differently (and have different atomic emission spectra) 2. how electrons are arranged Neils Bohr • Bohr worked with hydrogen and developed a new model of the atom: The Planetary Atomic Model Bohr’s Planetary Atomic Model: • electrons move around the nucleus in circular “orbits” (paths) much like planets orbit the sun • Each “orbit” has a certain level of energy smaller orbits = lower energy larger orbits = higher energy Energy . . . yeah baby! • electrons are able to change energy levels one at a time (like climbing the rungs of a ladder) • The LOWEST energy level available is called the ground state (it is the closest to the nucleus) • As an electron “jumps” from a low energy level to a higher one, it needs to absorb energy and become “excited” • like you would need energy to “jump” up high! electron that absorbed energy and is “excited” ground state nucleus • once an electron is “excited” and in a higher energy level, it can’t stay there because it’s not the “home orbit” • An electron will “drop” to a lower energy level • Every time an electron drops levels, it emits light – this is the light that we see when we put an element into a flame! • The electrons release the energy before they go “home” electron in ground state that emitted light energy nucleus Bohr worked to discover all of this, but keep in mind that he only worked with hydrogen! Scientists thinkin’ again . . . • If opposite charges attract, then why are negative electrons (that are lighter) not pulled into the positive nucleus????? • Well . . . It all has to do with energy – the energy of motion! • electrons actually “wiggle” as they move around the nucleus DeBroglie’s Wave Theory • DeBroglie proposed that electrons move like waves around the nucleus Heisenberg Heisenberg’s Uncertainty Principle: it is impossible to know the exact position of an electron (if they travel in waves) The Quantum Mechanical Model 1. developed by Schrödinger 2. atoms have a dense, positively charged nucleus 3. electrons surround the nucleus and are treated as waves, not particles 4. There is a GOOD probability of finding an electron in an atomic orbital **an atomic orbital is a threedimensional region around the nucleus (it’s not an orbit) You made it! Was it the worst day of your life?