blas et al 2005 funcecol 19 315-322.doc
advertisement

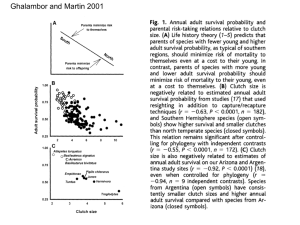
A multi-tier approach to identifying environmental stress in altricial nestling birds J. BLAS,*† R. B AOS,‡ G. R. BORTOLOTTI,* T. MA RC HANT* and F. HIRALDO‡ *Department of Biology, University of Saskatchewan, Saskatoon. Canada S7N 5E2, and ‡Department of Applied Biology, Estación Biológica de Doñana, C.S.I.C. Avda. Ma Luisa s/n, Pabellón del Perú, Apdo 1056, E-41013 Sevilla, Spain Summary 1. Birds respond to unpredictable events in the environment by releasing corticosterone, which triggers alternate responses to overcome stressful situations. However, it has been questioned whether altricial nestlings perceive and respond to ecological stressors. Although some studies show adrenal responsiveness in non-precocial species, others report a hyporesponsive period postulated to avoid deleterious effects of corticosterone. 2. To test whether the environmental surroundings of nestlings modulate their stress levels we used a multi-tier comparative approach of plasma corticosterone: (1) within an individual, (2) among nestlings within a brood hierarchy, (3) among broods within a colony and (4) among individuals from different colonies. 3. Nestlings reacted to our protocol by elevating their corticosterone. Baseline levels differed between colonies, and were higher in singleton nestlings compared with two- and three-chick broods, but there were no differences related to within-brood hierarchies, age or condition index. 4. Acute levels were higher in older birds, suggesting a developmental change in adrenal responsiveness. Body condition also explained acute levels but only in singleton nestlings, with higher concentrations in heavier birds. 5. Overall our results indicate that altricial nestlings do respond to a variety of environmental stressors, and variation in aspects of environmental quality (e.g. food supply, parental care) may be associated with the differences in stress. Key-words: Ciconia ciconia, corticosterone, ecological stress, habitat quality, parental quality Introduction The endocrine system orchestrates appropriate changes in the morphology, physiology and behaviour of organisms to specific life-history stages (Nelson 1995). Animals have evolved physiological strategies to cope with unpredictable components of the environment (Wingfield & Kitaysky 2002) and it is widely accepted that the facultative responses constitute a distinct emergency life-history stage (Wingfield et al. 1998). Among birds, sudden changes in weather conditions, famine, droughts or predation episodes activate the hypothalamus– pituitary–adrenal cortex (HPA) axis (Astheimer, Buttemer & Wingfield 1995; Romero 2000). The resulting secretion of glucocorticosteroids triggers alternate behavioural and physiological responses that temporarily suspend ‘non-essential’ activities for immediate survival, †Author to whom correspondence should be addressed. E-mail: julio@ebd.csic.es/julioblas@terra.es and promotes adaptive changes that maximize the possibility of surviving the perturbation and recovering homeostasis in the best possible physical condition (Silverin 1998a). A theoretical framework has been developed to explore the ecological bases of stress as well as underlying endocrine mechanisms, and birds have been used extensively as models (e.g. Wingfield & Silverin 2002). In spite of the substantial progress during the past decade, most field investigations have focused on adults, with fewer than 20 studies on nestlings having been published during the past 20 years. Only recently have ecological and behavioural endocrinologists been paying more attention to the stress responses of nest-bound birds (e.g. Schwabl 1999; Sims & Holberton 2000; Kitaysky et al. 2001a; Tarlow, Wikelski & Anderson 2001; Love, Bird & Shutt 2003a; Mullner, Linsenmair & Wikelski 2004). Given that altricial modes of development imply the inability to locomote during early stages, and parental care is crucial for providing food, heat, shelter and protection from predators, it has been proposed that any adrenocortical responsiveness would impair the individual’s ability to behaviourally overcome the stressful situation (Sims & Holberton 2000). In addition, the potential damage caused by chronic exposure to high corticosterone levels (Kitaysky et al. 2003) has been a major argument to suggest that young birds suppress the HPA axis, and that there is no adaptive value of a glucocorticoid response to environmental stressors (Romero, Soma & Wingfield 1998; Sims & Holberton 2000). Contrary to the latter hypothesis, stress responses may be beneficial for developing nestlings in spite of their parental dependence, and environmental stressors (Kitaysky et al. 2001a; Mullner et al. 2004), or withinbrood social interactions (e.g. Tarlow et al. 2001) may change glucocorticoid release. Although parents buffer environmental perturbations, the growing birds still have to face variation in food availability, sibling competition, parasites, predators and, eventually, adverse weather conditions. Furthermore, increases in corticosterone promote begging (Kitaysky et al. 2001b) and aggressive behaviours (Kitaysky et al. 2003), and modulate metabolic rates (Buttemer, Asteimer & Wingfield 1991), all of which should be of adaptive value for nestlings under stressful circumstances. Few studies have tested whether potential ecological stressors such as differences in the environment surrounding the nestlings, or attributes related to parental quality, affect the adrenocortical response (but see Mullner et al. 2004). This research gap may be because most investigations have been performed under captive conditions (e.g. Schwabl 1999; Kitaysky et al. 2003; Love, Bird & Shutt 2003b), have limited their focus to ontogeny and developmental processes (Schwabl 1999; Sims & Holberton 2000; Love et al. 2003a), or to a specific proximate cause of perturbation such as food-stress (Kitaysky et al. 1999a, 2001a). Here, we test whether differences in the environment surrounding nestlings modulate their stress hormone levels using a multi-tier approach: (1) within an individual nestling along the time course of a standardized handling and restraint protocol, (2) among nestlings differing in their position within a brood hierarchy (i.e. sharing the same nest and parents), (3) among broods of different size within a breeding colony (i.e. sharing the same habitat) and (4) among individuals reared in different local environments within the same population. If the adrenocortical response to stress is suppressed during the nestling stage, we predict no effect of the different ecological conditions of brood hierarchy, brood size and locale on circulating corticosterone levels, as well as a lack of elevated corticosterone in response to capture and handling. If, on the contrary, nestlings have a functional HPA axis and respond to ecological stressors, then all of these sources of variation are plausible. As a study model, we used wild nestlings of European White Stork (Ciconia ciconia, Linnaeus), a large altricial wading bird. Materials and methods STUDY SPECIES AND STUDY AREAS The White Stork is a large (2200 – 4400 g) long-lived, monogamous bird that breeds from North Africa to northern Europe (del Hoyo, Elliot & Sargatal 1994). At Iberian latitudes it has a prolonged reproductive season (from February to July), and breeds solitarily or colonially in open nests on trees, cliffs, buildings and power poles. The chicks hatch after 33 –34 days of incubation, and depend on adults for the delivery of food until they leave the nest at 60 – 90 days of age ( Redondo, Tortosa & Arias de la Reina 1995). Fieldwork was conducted in two colonies in southern and mid-western Spain during 2000. The southernmost colony (Sevilla) was located beside the Guadalquivir marshes in the Dehesa de Abajo natural area, while the northernmost colony (Cáceres, 230 km away), was in a scattered oak forest surrounded by crop fields in Aldea del Cano. The stork population is continuous between sites, and there is no evidence suggesting genetic differences between locales. However, the distance between our colonies is large enough to ensure that there is no overlap in the use of habitat during the breeding season, as breeders usually feed near the nesting site (Cramp & Simmons 1980). FIELD PROCEDURES AND BLOOD SAMPLING During the first week of June prior to blood sampling, nests at both study sites were checked to select a random sample of different brood sizes. Chosen nests (n = 40) could have one, two or three chicks and were similarly distributed between locales. Blood sampling (n = 70 birds) comprised either the single nestling or both the oldest and the youngest birds within a brood in the case of two- and three-chick nests. Several teams composed of three people entered the colonies and followed the same strategy for approaching nests. Teams worked concurrently at different nests in the same tree or at nearby trees, and followed a predetermined route to avoid causing additional stress to breeding pairs or nestlings. Nestlings underwent a standardized capture, handling and restraint protocol known to elicit an increase in circulating corticosterone in birds ( Wingfield & Kitaysky 2002). After accessing a nest with a ladder, we captured the storks by hand and immediately brought them down to the ground. The first blood sample was taken within the first minute of capture. The synchronized work of several persons allowed simultaneous sampling of two young per nest in the case of two- or three-chick nests (n = 17 and 13 broods, respectively). Additional samples were collected from the brachial vein at 2, 10, 30 and 45 min after capture, and nestlings were held in individual boxes between consecutive samples. After blood sampling, nestlings were banded and their wing chord and body mass were measured before returning them to the nest. All field activities were performed between 07:00 and 11:00 h to minimize potential diel effects. Blood samples were kept on ice until centrifuged (3000 r.p.m. for 10 min) the same day, and plasma was frozen and stored at −80 °C until corticosterone measurement. The relative position of siblings in the brood hierarchy was categorized as either ‘senior’ for the oldest, and ‘junior’ for the youngest. Given that brood reduction in white storks normally affects the youngest chicks (Sasvári, Hegyi & Péczely 1999), we assumed that single nestlings were senior, although for statistical analyses (see later) the effect of brood hierarchy was also tested excluding singletons. Reproductive activities at Sevilla were monitored periodically between March and July 2000 as part of a long-term study (Jovani & Tella 2004). By checking nest contents at least three times we gathered data on clutch size, hatching success and fledging success of individual pairs. Nests with different brood sizes at blood sampling, did not differ in clutch size at the beginning of the breeding season (average ± SE clutch size = 3·43 ± 0·08, n = 114 nests, F 2,111 = 0·961, P = 0·39). Nestling age (estimated following Negro et al. 2000) ranged from 24 to 59 days, and was always above the time at which maximum thermoregulatory ability is acquired (Tortosa & Castro 2003; Jovani & Tella 2004). A body condition index was calculated as the individual’s residual value from a reduced major axis regression of log body mass on log wing chord, following Green (2001). Our condition estimate fits the required assumptions of strong linearity between lg mass and lg size (Pearson r = 0·925, P < 0·001) and independence of body size (Pearson r = 0·126, P = 0·3). costerone concentrations in samples taken within 1 min and after 45 min of handling and restraint were used as an estimate of the baseline and acute stress levels, respectively. STATISTICAL TREATMENT Baseline and acute corticosterone levels were analysed separately through Generalized Linear Mixed Models (GLMMs, Littell et al. 1996), using the macro glimmix 8·0 for SAS (SAS 1997). GLMMs simultaneously account for both random effects, fixed effects and covariates. By including the nest as a random variable, we avoided pseudoreplication derived from the lack of independence in values from nestlings within the same brood. In addition, mixed GLMs offer the possibility of assessing the amount of variation (i.e. deviance) explained by the resulting models (McCullagh & Nelder 1989; Littell et al. 1996). Initial models were adjusted to a normal distribution of errors and an identity link function was used. The models were constructed by a stepwise backward procedure, where the least significant terms were sequentially removed (McCullagh & Nelder 1989; Crawley 1993). Initial effects comprised colony site, brood size and brood hierarchy as fixed factors, age and body condition as covariates, and all the two-way interactions. Nest identity was included as a random factor. Final (i.e. minimum adequate) models only retained those terms showing a significant effect on the corticosterone values at a >5% rejection probability. Results BASELINE CORTICOSTERONE LEVELS CORTICOSTERONE DETERMINATION Plasma corticosterone concentration was determined utilizing a radioimmunoassay (RIA) described previously (Wayland et al. 2002). Measurements were performed on reconstituted ethyl ether extracts of the plasma samples. The efficiency of organic extraction was estimated as described in Wayland et al. (2002) and found to be consistently greater than 90% for the stork samples. Each extract was measured in the RIA in duplicate. Serial dilutions of the nestling stork plasma extracts produced displacement curves parallel to that of the purified corticosterone standards. The minimum detection limit of the assay was 0·10 ng ml−1, but our samples were diluted several-fold so as to fall near the mid-range (0·43 ng ml−1) of the standard curve. Samples were measured over a total of nine separate assays; the intra- and interassay coefficients of variation were 7·1% and 8·0%, respectively. Assay precision was assessed by including an internal control with a known amount of corticosterone (1·60 ng ml−1) in each RIA; the measured value of the internal control was 1·68 ± 0·074 (mean ± SD, n = 9) ng ml −1. A preliminary survey of the corticosterone time-course response along the complete serial sampling period indicated that maximum levels were attained after 45 min. Therefore, corti- rood size and colony location showed statistically significant effects on baseline corticosterone levels ( Table 1). Overall plasma resting values were more than two-fold higher in Cáceres than in Sevilla (Fig. 1). This difference was not a consequence of the handling protocol, as all birds were bled within the first minute following capture, and the time from first entry to the colony to Fig. 1. Baseline and acute levels of plasma corticosterone as a function of the colony site ( = Sevilla colony; D = Cácere colony). Bars represent mean values ± 1 standard error (n = 35 per locality and sampling time). *P < 0·05; ns P > 0·05. Table 1. Summary of results from the GLMM explaining baseline plasma corticosterone concentration in young white storks (n = 70 birds). The estimated effect and standard error as well as F-values and associated probabilities are shown for those variables that significantly improved the fit of the model. For those terms that were excluded from the model during the backwards procedure, F and P-values before exclusion are also shown. Data were modelled following a normal distribution of errors and an identity link function, and nest identity was treated as a random factor. The final model explained 49·1% of the initial deviance in corticosterone values Effect Estimate Standard error F-value (df ) P Intercept Colony Brood size 13·4807 14·4084a 11·6051b −2·2264c – – – – – – – – – – – – – 2·0216 2·1505 3·3394 2·3441 – – – – – – – – – – – – – 6·67d (36) 44·89 (1, 30) 9·27 (2, 30) <0·0001 <0·0001 0·0007 0·13 (1, 25) 0·55 (1, 27) 0·48 (1, 29) 1·88 (2, 24) 0·63 (1, 20) 0·30 (1, 21) 0·01 (1, 18) 0·87 (1, 23) 0·93 (2, 22) 3·09 (2, 28) 0·27 (1, 19) 3·48 (1, 24) 3·63 (1, 26) 0·7228 0·4667 0·4962 0·1740 0·4367 0·5924 0·9072 0·3610 0·4098 0·0612 0·6083 0·0744 0·0680 Brood hierarchy Age Body condition Colony × Brood size Colony × Brood hierarchy Colony × Age Colony × Body condition Brood size × Brood hierarchy Brood size × Age Brood size × Body condition Brood hierarchy × Age Brood hierarchy × Body condition Age × Body condition a Estimate corresponds to Cáceres colony. Estimate corresponds to brood size = 1. c Estimate corresponds to brood size = 2. d t-test. b individuals’ blood sampling was not related to baseline corticosterone values (Pearson r = 0·38, P = 0·76, n = 70). The significant effect of brood size was analysed by performing post hoc contrasts on the final model. Nestlings from brood sizes of one (32·3 ± 17·3 ng ml−1, n = 10) showed higher baseline levels than nestlings from twochick (18·9 ± 8·7 ng ml−1, n = 34, post hoc contrast F1,30 = 18·36; P = 0·0002) and three-chick nests (20·1 ± 12·2 ng ml−1, n = 26, post hoc contrast F1,30 = 12·08; P = 0·0016); the latter two were not significantly different from each other ( post hoc contrast F1,30 = 0·90; P = 0·35, Fig. 2). Neither brood hierarchy nor age or body condition index accounted for differences in baseline values (P > 0·1, Table 1), and there were no significant differences in condition index between nestlings that differed in brood size ( F2,67 = 1·05, P = 0·35), or in colony ( F1,68 = 0·604, P = 0·44). The final model explained 49·1% of the deviance in baseline levels, with colony site alone explaining 34·4%, while brood size alone explained 13·9%. When the analysis was restricted to the sample of nests with more than one young, brood hierarchy did not influence corticosterone levels. Senior and junior nestlings shower similar baseline concentrations (F1,29 = 0·01; P = 0·99, Fig. 3), but the difference between colonies was again significant (F1,29 = 33·99; P < 0·0001). The interaction between brood size and brood hierarchy was not significant (F1,28 = 0·03; P = 0·86). ACUTE CORTICOSTERONE LEVELS After 45 min of handling and restraint, corticosterone values increased around two-fold compared with base- Fig. 2. Baseline and acute levels of plasma corticosterone as a function of the brood size. Bars represent mean values ± 1 standard error. Sample sizes are shown above bars. *P < 0·05; ns P > 0·05. line levels (baseline 21·3 ± 12·3 ng ml−1; acute 39·1 ± 11·3 ng ml−1; repeated measures t = −9·59; P < 0·001; n = 70). Acute corticosterone levels did not differ between colonies (Fig. 1) nor were they affected by brood hierarchy (Table 2). Age had a positive effect on acute levels (F1,27 = 17·91; P = 0·0002), and the interaction term Brood size × Body condition was also significant (F2,27 = 3·75; P = 0·036, Table 2). When the effect of body condition was separately tested in each of the brood size groups, only singletons showed a significant Table 2. Summary of results from the GLMM explaining stress-induced (i.e. after 45 min of restraint) plasma corticosterone concentration in young White Storks (n = 70 birds). The estimated effect and standard error as well as F-values and associated probabilities are shown for those variables that significantly improved the fit of the model. For those terms that were excluded from the model during the backwards procedure, F and P-values before exclusion are also shown. Data were modelled following a normal distribution of errors and an identity link function, and nest identity was treated as a random factor. The final model explained 61·0% of the initial deviance in corticosterone values Effect Estimate Standard error Intercept Colony Brood size 15·9074 – 2·1191b −1·6195c – 0·5396 −52·4038 – – – – – – 155·19b −9·0289c – – – 5·7102 – 3·7680 2·8363 – 0·1275 54·1859 – – – – – – 72·9620 68·7838 – – – Brood hierarchy Age Body condition Colony × Brood size Colony × Brood hierarchy Colony × Age Colony × Body condition Brood size × Brood hierarchy Brood size × Age Brood size × Body condition Brood hierarchy × Age Brood hierarchy × Body condition Age × Body condition F-value (df ) P 2·79a (36) 0·89 (1, 25) 0·59 (2, 27) 0·0085 0·3543 0·5626 0·02 (1, 26) 17·91 (1, 27) 0·02 (1, 27) 1·17 (2, 19) 0·03 (1, 19) 0·30 (1, 21) 0·01 (1, 22) 0·78 (1, 24) 0·43 (2, 20) 3·75 (2, 27) 0·8889 0·0002 0·8961 0·8482 0·8703 0·5927 0·9273 0·3863 0·6561 0·0365 3·30 (1, 25) 0·01 (1, 18) 0·03 (1, 23) 0·0813 0·9312 0·8560 a t-test. Estimate corresponds to brood size = 1. c Estimate corresponds to brood size = 2. b (senior: 40·4 ± 9·2 ng ml−1; n = 30; junior 37·0 ± 12·2 ng ml−1; n = 30; Fig. 3). Discussion Fig. 3. Baseline and acute levels of plasma corticosterone as a function of brood hierarchy. Birds from nests of broods sizes of two and three are grouped together. Senior birds (n = 30) were the oldest individuals within a brood, and junior birds (n = 30) were the youngest. Error bars represent ± 1 standard error of the mean (ns P > 0·05). and positive association with acute corticosterone levels (F1,8 = 8·05; P = 0·0219), but neither two-chick nor three-chick broods revealed any significant effect (Brood size = 2: F1,16 = 2·04, P = 0·1724; Brood size = 3: F1,12 = 1·20, P = 0·2941). Overall acute levels did not differ between nestlings from different brood sizes (F2,27 = 0·59, P = 0·5626, Table 2). Among brood sizes of two and three chicks, senior and junior siblings showed similar acute corticosterone concentrations Our results are clearly contrary to the perception of altricial nestlings as totally dependent organisms whose adrenocortical responsiveness is suppressed (e.g. Sims & Holberton 2000). After 45 min of handling and restraint, corticosterone levels were significantly higher than baselines in the same individuals. By extension of this response to handling, there is evidence that other exogenous (ecological) stressors from the environment also have the potential to change corticosterone secretions in the early stages of life, as indicated by recent studies in other non-precocial bird species (Mullner et al. 2004). Our finding would hardly be a consequence of a maladaptive physiological response, as one would expect strong selection against individuals with impaired glucocorticoid secretion, given the known negative repercussions of an over-exposure to corticosterone on growth, learning and developmental processes (Kitaysky et al. 2003; Hayward & Wingfield 2004). Baseline corticosterone titres from Cáceres were consistently higher than those from Sevilla. This result accounted for a considerable proportion of the variance, and suggests that environmental differences between locales affect circulating glucocorticoids (see Mullner et al. 2004). The Sevilla colony was located beside a marsh (one of the most productive habitats in the world, Odum 1961), whereas the Cáceres colony was adjacent to a stream and crop fields. There was probably a higher availability of fish, amphibians and aquatic invertebrate prey at the Sevilla colony, all of which normally constitute the main food resources for breeding White Storks (Cramp & Simmons 1980; Negro et al. 2000). More importantly, the introduced American Crayfish (Procambarus clarkii) is a plague in Sevilla marshes (Gutiérrez-Yurrita et al. 1999), and is a unique example of a super-abundant prey resource for the local community of breeding wading birds (Hiraldo & Tablado 2003). In fact, crayfish represents the only prey remains in 55% of White Stork nests in Sevilla (Negro et al. 2000). The extraordinary food supply has often been used to explain the population growth of White Storks at the Sevilla colony even at a time when the overall Spanish population of White Storks was declining (Senra & Alés 1992; Hiraldo & Tablado 2003). At least two studies have shown that reduced abundance of food resources triggers baseline increases of the stress levels of wild adult birds (in passerines, Marra & Holberton 1998; and sea-birds, Kitaysky, Wingfield & Piatt 1999b), and this relationship is well supported by experiments with developing nestlings (Kitaysky et al. 1999a; Kitaysky et al. 2001a). Another environmental difference between colonies was the population size and density of breeders, as it was considerable higher in Sevilla (about 300 pairs) than in Cáceres (about 50 pairs). Although there is evidence showing that the density of conspecifics affects individual levels of stress hormones (Silverin 1998b), the direction of those results was opposite to the pattern we found, as corticosterone levels were higher in chicks from the less populated Cáceres colony. Recent work has also shown that social hierarchies within a brood correlate with between-siblings differences in circulating glucocorticosteroids (Nuñez de la Mora, Drummond & Wingfield 1996; Schwabl 1999; Ramos-Fernández et al. 2000; Tarlow et al. 2001; Love et al. 2003b; but see Sockman & Schwabl 2001; Love et al. 2003a), and that corticosterone may play a role in the aggressive behaviours of chicks ( Kitaysky et al. 2003). For example, in the obligate siblicidal Galapagos Nazca Booby (Sula granti, Tarlow et al. 2001) and the facultative siblicidal Blue-Footed Booby (Sula nebouxii, Ramos-Fernández et al. 2000), the subordinate chick has higher circulating corticosterone than its dominant sibling. Furthermore, single chicks tend to have either lower corticosterone titers than two-brood nestlings (Tarlow et al. 2001), or circulating levels similar to dominant birds (Nuñez de la Mora et al. 1996), suggesting a role of this hormone in competitive social behaviours. Contrary to this pattern, we found no differences associated with brood hierarchy, as senior and junior birds showed similar titres. In addition, stress hormone levels were similar among two- and three-chick broods and statistically higher in single stork nestlings. If circulating levels of corticosterone respond to social interactions, we may expect an increase with brood size parallel to the increasing chances of sibling aggression. Our results suggest that corticosterone may not play a role in the organization of within-broods social hierarchies in white storks. Nevertheless, siblicidal behaviours in storks lack the relevance recorded in the booby species, as aggressive interactions only take place close to the age of fledging, when mortality rarely occurs (Tortosa & Redondo 1992; Sasvári et al. 1999). It is important to note that single nestlings in the present study were the result of breeding attempts that suffered a higher loss of eggs or chicks, as nests of different brood sizes did not differ in clutch size at the beginning of the breeding season. It is therefore plausible that single nestlings were reared by parents of low quality, for example, young or inexperienced breeders (Forslund & Pärt 1995). Other studies have reported negative associations between nestling mortality in White Storks and some measures of parental quality such as the body mass of adults and their food delivery rate (Sasvári et al. 1999). A lower feeding efficiency among poor-quality breeders would explain both the increased nestling mortality and the elevated corticosterone in the surviving chick. Furthermore, reduced nest attendance by parents would result in the developing young being more exposed to the elements, a factor known to elicit corticosterone increases in birds (Romero 2000; Wingfield & Kitaysky 2002), and to contribute to nestling mortality in White Storks (Jovani & Tella 2004). Among adult birds, it is well known that reduced food supply elevates baseline corticosterone, which in turn promotes adaptive behavioural changes such as increased foraging activity and rate of ingestion (Wingfield, Schwabl & Mattocks 1990). Nestlings are limited in their ability to perform these behaviours, and in fact, this has been one of the arguments for the lack of any adaptive value of nestling adrenocortical responsiveness (Sims & Holberton 2000). However, the pioneering work of Kitaysky and colleagues demonstrated that corticosterone increases facilitate begging in nestling gulls (Kitaysky, Wingfield & Piatt 2001b; Kitaysky et al. 2003). Such behaviour is unquestionably an adaptive response to food shortages, which is functionally equivalent to foraging in mature birds. The positive association between condition index and acute corticosterone levels observed in single stork nestlings is contrary to previous findings. Experimental studies have shown that food-restricted chicks have higher baseline and acute stress-induced levels compared with chicks fed ad libitum. Consequently, fat reserves and body mass are usually found to be negatively correlated with adrenocortical secretion in both chicks (Heath & Dufty 1998; Kitaysky et al. 2001a) and adult birds (Wingfield & Silverin 2002). This relationship may reflect the ability of birds in good condition to mobilize fat for energy, while birds in poor condition must mobilize protein. However, Silverin, Advidsson & Wingfield (1997) found opposite associations for female Willow Warblers (Phylloscopus trochilus) breeding at different latitudes. While in northern locations body mass was negatively related to acute levels, in southern sites the association was positive. Although genotypic differences may explain the findings in Willow Warblers, this is not a plausible explanation for the between-broods differences reported in our population of storks, and the functional explanation for higher stress-induced corticosterone levels in birds in good condition remains unclear. Baseline and acute postcapture corticosterone levels showed different patterns of variation. Acute responses were affected by nestling age and condition, and not by colony, brood size or hierarchy. Conversely, baseline levels changed with the environmental variables but were independent of age. According to the developmental hypothesis (Kitaysky et al. 2003), development of HPA responsiveness in a nestling should parallel the individual’s abilities to behaviourally and physiologically overcome stressors. The varied nature of the stimuli potentially inducing stress implies that some adaptive responses may be developed during early life stages, while others require longer development. For example, one would predict that adrenal responsiveness to food stress would be developed in early stages, as it plays a major role in begging behaviour and therefore in offspring–adult communication to overcome the perturbation. On the other hand, our capture and restraint protocol is a perturbation of a different nature (Canoine et al. 2002), possibly of higher magnitude and requiring more drastic responses (e.g. ‘fight or flight’, Wingfield & Ramenofsky 1999; Wingfield & Silverin 2002). The postcapture age pattern on corticosterone levels may thus reflect a progressive behavioural readiness to face short-term and drastic perturbations. Acknowledgements We thank J. C. Nuñez, who kindly helped to find the Cáceres colony and provided housing during the field activities. R. Jovani and J. L. Tella provided information on reproductive parameters from the Sevilla colony, and two anonymous referees made comments that considerably improved earlier drafts. We are also grateful to the many people that helped during field sampling, especially A. Román, A. Tamayo and J. Ramirez from SEO-Málaga, and also M. C. Quintero, J. L. Arroyo, B. Jiménez, R. Tobar, C. Alonso, R. Álvarez, R. Rodríguez and N. Pastor. This work was supported by EGMASA and the European Community in a i3P grant to J.B., the Spanish Ministerio de Educación Cultura y Deporte in a Postdoctoral Fellowship to J.B. and a Predoctoral FPU fellowship to R.B., and a NSERC grant to G.R.B. All birds were captured and handled according to Spanish law. The Consejería de Agricultura y Medio Ambiente de Cáceres, Consejería de Medio Ambiente de Andalucía, and Ayuntamiento de Puebla del Río provided permits. References Astheimer, L.B., Buttemer, W.A. & Wingfield, J.C. (1995) Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Hormones and Behavior 29, 442– 457. Buttemer, W.A., Asteimer, L.B. & Wingfield, J.C. (1991) The effect of corticosterone on standard metabolic rates of small passerine birds. Journal of Comparative Physiology B 161, 427– 431. Canoine, V., Hayden, T., Rowe, K. & Goymann, W. (2002) The stress response of European stonechats depends on the type of stressor. Behavior 139, 1303 –1311. Cramp, S. & Simmons, K.E.L. (1980) The Birds of the Western Paleartic. Oxford University Press, Oxford. Crawley, M.J. (1993) GLIM for Ecologists. Blackwell Scientific Publications, Cambridge. Forslund, P. & Pärt, T. (1995) Age and reproduction in birds: hypotheses and tests. Trends in Ecology and Evolution 10, 374 –378. Green, A.J. (2001) Mass / length residuals: measures of body condition or generators of spurious results? Ecology 82, 1473 –1483. Gutiérrez-Yurrita, P.J., Martínez, J.M., Bravo, M.A., Ilheu, M., Bernardo, J. & Montes, C. (1999) The status of crayfish populations in Spain and Portugal. Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation? (eds D.M. Holdich & F. Gherardi), pp. 161–192. Crustacean Issues, Vol. 11. A.A. Balkema, Rotterdam. Hayward, L.S. & Wingfield, J.C. (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. General and Comparative Endocrinology 135, 365–371. Heath, J.A. & Dufty, A.M. Jr (1998) Body condition and the adrenal stress response in captive American kestrels juveniles. Physiological Zoology 71, 6–73. Hiraldo, F. & Tablado, Z. (2003) Efectos del cangrejo rojo americano sobre la comunidad de vertebrados de Doñana. Estación Biológica de Doñana, CSIC, Sevilla. del Hoyo, J., Elliot, A. & Sargatal, J. (1994) Handbook of the Birds of the World, Lynx Edicions, Barcelona. Jovani, R. & Tella, J.L. (2004) Age-related environmental sensitivity and weather mediated nestling mortality in white storks (Ciconia ciconia). Ecography 27, 611– 618. Kitaysky, A.S., Piatt, J.F., Wingfield, J.C. & Romano, M. (1999a) The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. Journal of Comparative Physiology B 169, 303 –310. Kitaysky, A.S., Wingfield, J.C. & Piatt, J.F. (1999b) Dynamics of food availability, body condition and physiological stress response in breeding Black-Legged Kittiwakes. Functional Ecology 13, 577–584. Kitaysky, A.S., Kitaiskaia, E.V., Wingfield, J.C. & Piatt, J.F. (2001a) Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. Journal of Comparative Physiology B 171, 701–709. Kitaysky, A.S., Wingfield, J.C. & Piatt, J.F. (2001b) Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behavioral Ecology 12, 619 – 625. Kitaysky, A.S., Kitaiskaia, E.V., Piatt, J.F. & Wingfield, J.C. (2003) Benefits and costs of increased levels of corticosterone in seabird chicks. Hormones and Behavior 43, 140 –149. Littell, R.C., Stroup, W.W., Milliken, G.A. & Wolfinger, R.D. (1996) Generalized Linear Mixed Models. SAS Institute Inc., Cary, NC. Love, O.P., Bird, D.M. & Shutt, L.J. (2003a) Corticosterone levels during post-natal development in captive American kestrels (Falco sparverius). General and Comparative Endocrinology 130, 135 –141. Love, O.P., Bird, D.M. & Shutt, L.J. (2003b) Plasma corticosterone in American kestrel siblings: effects of age, hatching order, and hatching asynchrony. Hormones and Behavior 43, 480 – 488. . Marra, P.P. & Holberton, R.L. (1998) Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116, 284 –292. McCullagh, P. & Nelder, J.A. (1989) Generalized Linear Models, 2nd edn. Chapman & Hall, New York. Mullner, A., Linsenmair, K.E. & Wikelski, M. (2004) Exposure to ecotourism reduces survival and affects stress response in hoatzin chicks (Opisthocomus hoazin). Biological Conservation 118, 549 –558. Negro, J.J., Tella, J.L., Blanco, G., Forero, M.G. & GarridoFernández, J. (2000) Diet explains interpopulation variation of plasma carotenoids and skin pigmentation in nestling white storks. Physiological and Biochemical Zoology 73, 97–101. Nelson, R.J. (1995) An Introduction to Behavioral Endocrinology, Sinauer Associates Inc., Sunderland, MA. Nuñez de la Mora, A., Drummond, H. & Wingfield, J.C. (1996) Hormonal correlates of dominance and starvation-induced aggression in chicks of the blue-footed booby. Ethology 102, 748 –761. Odum, E.P. (1961) The role of tidal marshes in estuarine production. Conservationist (NY State Conservancy) 15, 12–15. Ramos-Fernández, G., Núñez-de la Mora, A., Wingfield, J.C. & Drummond, H. (2000) Endocrine correlates of dominance in chicks of the blue-footed booby (Sula nebouxii): testing the challenge hypothesis. Ethology, Ecology and Evolution 12, 27–34. Redondo, T., Tortosa, F.S. & Arias de la Reina, L. (1995) Nest-switching and alloparental care in colonial white stork. Animal Behaviour 49, 1097–1110. Romero, L.M. (2000) Effects of weather on corticosterone responses in wild free-living passerine birds. General and Comparative Endocrinology 118, 113 –122. Romero, L.M., Soma, K.K. & Wingfield, J.C. (1998) The hypothalamus and adrenal regulate modulation of corticosterone release in redpolls (Carduelis flammea – an arcticbreeding song bird). General and Comparative Endocrinology 109, 347–355. SAS (1997) SAS/STAT Software: Changes and Enhancements Through Release 6·12. SAS Institute Inc., Cary, NC. Sasvári, L., Hegyi, Z. & Péczely, P. (1999) Brood reduction in white storks mediated through asymmetries in plasma testosterone concentrations in chicks. Ethology 105, 569 – 582. Schwabl, H. (1999) Developmental changes and among-sibling variation of corticosterone levels in an altricial avian species. General and Comparative Endocrinology 116, 403 – 408. Senra, A. & Alés, E.E. (1992) The decline of the white stork Ciconia ciconia population of western Andalusia between 1976 and 1988: causes and proposals for conservation. Biological Conservation 61, 51–57. Silverin, B. (1998a) Stress responses in birds. Poultry and Avian Biology Reviews 9, 153 –168. Silverin, B. (1998b) Territorial behaviour and hormones of pied flycatchers in optimal and suboptimal habitats. Animal Behaviour 56, 811– 818. Silverin, B., Ardvidsson, B. & Wingfield, J.C. (1997) The adrenocortical responses to stress in breeding Willow Warblers, Phylloscopus trochilus. in Sweden: effects of latitude and gender. Functional Ecology 11, 376 –384. Sims, C. & Holberton, R. (2000) Development of the corticosterone stress response in young northern mockingbirds (Mimus polyglottos). General and Comparative Endocrinology 119, 193 –201. Sockman, K.W. & Schwabl, H. (2001) Plasma corticosterone, in nestling American kestrels: effects of age, handling stress, yolk androgens, and body condition. General and Comparative Endocrinology 122, 205 –212. Tarlow, E.M., Wikelski, M. & Anderson, D.J. (2001) Hormonal correlates of Siblicide in Galápagos Nazca boobies. Hormones and Behavior 40, 14 –20. Tortosa, F.S. & Castro, F. (2003) Development of thermoregulatory ability during ontogeny in the white stork Ciconia ciconia. Ardeola 50, 39 – 45. Tortosa, F.S. & Redondo, T. (1992) Motives for parental infanticide in white storks Ciconia ciconia. Ornis Scandinavica 23, 185 –189. Wayland, M., Gilchrist, H.G., Marchant, T., Keating, J. & Smits, J.E. (2002) Immune function, stress response, and body condition in arctic-breeding common eiders in relation to cadmium, mercury and selenium concentrations. Environmental Research Section A 90, 47– 60. Wingfield, J.C. & Kitaysky, A.S. (2002) Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integrative and Comparative Biology 42, 600 – 609. Wingfield, J.C. & Ramenofsky, M. (1999) Hormones and the behavioral ecology of stress. Stress Physiology in Animals (ed. P.H.N. Balm). Sheffield Academic Press, Sheffield. Wingfield, J.C. & Silverin, B. (2002) Ecophysiological studies of hormone-behavior relations in birds. Hormones, Brain and Behavior (eds D.W. Pfaff, A.P. Arnold, A.M. Etgen, S.E. Fahrbach & R.T. Rubin), Vol. 2, pp. 587– 647. Academic Press, San Diego. Wingfield, J.C., Schwabl, H. & Mattocks, P.W.J. (1990) Endocrine mechanisms of migration. Bird Migration (ed. E. Gwinner), pp. 232–256. Springer-Verlag, Berlin. Wingfield, J.C., Maney, D.L., Breuner, C.W., Honey, P.K., Jacobs, J.D., Lynn, S., Ramenofsky, M. & Richardson, R.D. (1998) Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. American Zoologist 38, 191–206. Received 7 August 2004; revised 27 December 2004; accepted 6 January 2005