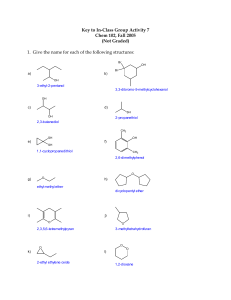
ORGANIC CHEMISTRY QUIZ 1) Which of the following is true about Thiols? A. They are alcohols that contain a sulfur atom in place of the oxygen atom. B. They are carboxylic acids that contain a sulfur atom in place of the carbon atom. C. They are a type of hydrocarbon that contains a sulfur atom in its structure. D. They are a type of polymer that contains sulfur in its backbone. 2) Which of the following functional groups is present in a thiol molecule? A. Carbonyl group B. Carboxyl group C. Hydroxyl group D. Sulfhydryl group 3) Which of the following is true about phenol? A. It is a type of alcohol. B. It is a type of carboxylic acid. C. It is a type of organic compound that contains a hydroxyl group (-OH) attached to an aromatic ring. D. It is a type of hydrocarbon that contains a nitrogen atom in its structure. 4) What is the general formula for ethers? A. R-OH B. R-O-R' C. R-COOH D. R-CHO 5) Which of the following is a characteristic property of ethers? A. They have a high boiling point. B. They are soluble in water. C. They have a sweet odor. D. They are strong acids. 6) Phenol is commonly used in the production of which of the following materials? A. Plastics B. Textiles C. Paper D. Metals 7) Draw the structure of each of the following compounds: a. Phenol b. 4-Nitrophenol c. 2,4-Dimethylphenol d. 3-Chloro-5-methylphenol e. 2,3,5-Trichlorophenol 8) A group of scientists conducted an experiment to investigate the flammability of diethyl ether. They first set up a fume hood to ensure that any potentially harmful vapors would be safely ventilated. They then filled a small glass dish with a small amount of diethyl ether and placed it on a heat-resistant surface. Next, they used a long-handled lighter to ignite the ether vapor above the dish. They observed a bright blue flame that quickly spread across the surface of the dish. Once the ether was consumed by the flame, the flame quickly extinguished and the dish was allowed to cool. The scientists then cleaned up any remaining ether and materials used in the experiment, and disposed of them properly. This experiment demonstrated that diethyl ether is highly flammable and can easily ignite in the presence of an ignition source. It also highlights the importance of proper safety precautions when working with potentially dangerous substances. What did a group of scientists observe when they conducted an experiment to investigate the flammability of diethyl ether? a) A green gas was produced when the ether was ignited with a long-handled lighter. b) The ether vapor did not ignite when exposed to an ignition source. c) A bright blue flame quickly spread across the surface of the dish when the ether was ignited with a long-handled lighter. d) The dish shattered when the ether was heated in the glass dish. 9) Name the IUPAC names of the following thiols: a. CH3CH2SH b. CH3CH2CH2SH c. CH3SCH2CH2CH3 d. (CH3)2CHSH 10) Which of the following compounds has the highest boiling point, and why? a) Diethyl ether b) Ethyl mercaptan (ethanethiol) c) Phenol (hydroxybenzene)