Lecture 6.pptx
advertisement

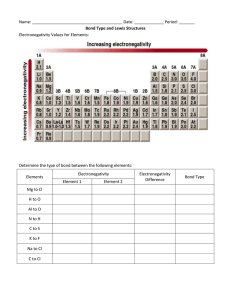
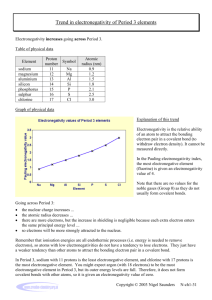
Lecture 6 ELECTRONEGATIVITY AND BOND TYPE Electronegativity is the power of an atom to attract electrons to itself in a chemical bond. Electronegativity increases from left to right along a period, and generally decreases down groups in the periodic table. Estimation of electronegativity Pauling electronegativity is based on bond energies, using the empirical observation that bonds between atoms with a large electronegativity difference tend to be stronger than those where the difference is small. This scale was historically the first to be devised and although it lacks a firm theoretical justification is still widely used. Mulliken electronegativity is the average of the first ionization energy and the electron affinity of an atom, reflecting the importance of two possibilities in bond formation, losing an electron or gaining one. The scale has the advantage that electronegativity values can be estimated not only for the ground states of atoms, but for other electron configurations and even for polyatomic fragments. Allred-Rochow electronegativity is proportional to Zeff/r2, where Zeff is the effective nuclear charge of valence orbitals , and r the covalent radius of the atom. The value is proportional to the effective electrostatic attraction on valence electrons by the nucleus, screened by inner shell electrons. Electronegativity increases towards the right and decreases towards the bottom in the periodic table. It thus follows the same trend as atomic ionization energies). Elements in early groups have low values and are called electropositive. Elements of group 18 in early periods do not form any stable compounds, and so the most electronegative element is fluorine. Pauling electronegativity values for the elements H–K. Elements in the shaded region are metallic Periodic trends of electronegativity using the Pauling scale The bond type How the electronegtivities of two elements A and B (which may be the same) determine the type of bond formed between them? 1- When A and B are both electropositive they form a metallic solid, characterized by high electrical conductivity and a structure where each atom is surrounded by many others Metallic bonding involves the delocalization of electrons throughout the solid. The electrons are shared between atoms as in covalent bonding but in a less specific way and without the directional character of covalent bonds. 2- When A and B are both electronegative they form covalent compounds. These may consist of individual molecules (O2, H2O, etc. 3- When one atom is very electropositive and the other very electronegative, a solid compound is formed that is often regarded as ionic. In this picture there is a complete transfer of one or more electrons, giving cations of the electropositive element and anions of the electronegative one. . Bond polarity 1- A covalent bond between two atoms of the same element is described as homopolar. 2- one between different elements as heteropolar. 3- A polar bond is a covalent bond in which there is a separation of charge between one end and the other - in other words in which one end is slightly positive and the other slightly negative. Examples include most covalent bonds. The hydrogenchlorine bond in HCl or the hydrogen-oxygen bonds in water are typical. In general, the greater the difference in electronegativity between two atoms, the more polar the bond that will be formed between them, with the atom having the higher electronegativity being at the negative end of the dipole bond polarity describes the unequal sharing of electrons between two atoms, and is a feature of heteropolar bonds when the two elements concerned have a different electronegativity. The more electronegative atom draws electrons and thus acquires a partial negative charge, with the other atom becoming correspondingly positive. Quiz Why does electronegativity increase across a periode? Electronegativity increases across a period because the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly. Why does electronegativity fall as you go down a group? because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. electric dipole moment, the measure of the electrical polarity of asystem of charges. dipole moment – μ – (units of debyes D (3.336x10-30 C·m)): μ = Q·R where Q is the charge and R is the bond length