chapter 5b

ALKYNES
Alkynes are hydrocarbons that contain a carbon – carbon triple bond (with no functional groups, only substituents present).
They are characterized by molecular formula C n
H
2n-
2
.
They are said to be unsaturated (having triple bond).
They are also called aliphatic hydrocarbons ( chain compounds)
Relatively not common in nature compared to alkenes.
SOME IMPORTANT TERMS FOR
NAMING ALKYNES
For naming the alkynes sytematically first we have to study about the following four features:
(a) Root word
(b)Primary suffix
(c)Secondary suffix
(d)Prefix
ROOT WORD:
(PARENT CHAIN/MAIN CHAIN)
It means the carbon chain. Some commonly used root words in alkynes are :
Chain length Root Word
C1 Meth-
C2 Eth-
C3 Prop-
C4 But-
C5 Pent-
C6 Hex-
C7 Hept
C8 Oct-
C9 Non-
C10 Dec-
Primary Suffix:
The primary suffixes are added to the root word to show unsaturation(triple bond) in a carbon chain.
Name of Carbon Primary Suffix IUPAC
Chain name
Unsaturated -yne
(CH ≡ CH)
Alkyne
PREFIX
Prefixes are added to the root word to indicate the presence of one or more substituents on the main carbon chain. These prefixes include: fluoro(-F)
Chloro(-Cl) bromo(-Br)
Ido(-I)
Nitro(-NO
2
)
Alkoxy(-O-R)
Alkyl groups
Arrangement of Prefixes, Root word and Suffixes
The prefixes, root words, primary and secondary suffixes are arranged as follows while writing the name of an alkyne.
IUPAC name = Prefixes + Root word + Primary
Suffix + Secondary suffix
RULES FOR NAMING ALKENES
The base or parent chain of alkyne is determined by the longest chain of carbon atoms containing the triple bond in the formula. The longest chain may be bend or twist. It is seldom horizontal.
CH
3
-CH
2
-CH
2
-CH
2
-C
║ CH
3
-CH
2
-CH
2
C |
CH
3
|
CH
║
2
| CH
3
-CH
2
-CH-CH
2
-C
CH
3
7 CARBON CHAIN 8 CARBON CHAIN
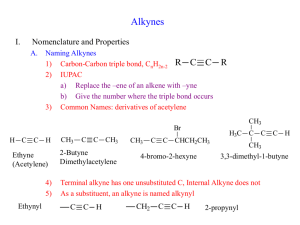
If the chain contain only triple bond and no other substituents, the chain is numbered starting from the
Carbon closest to the triple bond and the triple bond is given the lower number of its two triple-bonded carbon atoms.
CH ≡ C – CH
2
– CH
3
1- Butyne
OR
But – 1 – yne
•
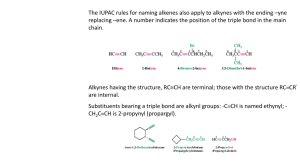
A compound with two triple bonds is a diyne.
A triyne has three triple bonds and a tetrayne has four bonds.
•
Numbers are used to specify the locations of the triple bonds.
CH≡C–C≡CH
1,3-butadiyne buta-1,3-diyne
If one substituent or functional group is attached to the parent chain, then numbering should start from the side to give minimum number to the sum of substituent and the triple bond. e.g.
1 CH
║
2 C 8 CH
3
7 CH
2
6 CH
2
| |
5 CH
2
4 CH
2
3 CH-CH
3
The name of this compound is 3- methy-l- octyne or
3- methyl-oct-1-yne. We will start the numbering from right side because from right side the sum of the numbers of carbon containing substituent and the triple bond is 1+3=4 but from left side it is 6+8=14.
If a double bond and triple bond are on the same parent chain, numbering will start from the side to give least sum of the number of carbons containing double and triple bonds but the structure will be named as an alkyne.
1 CH
2
═ 2 CH – 3 C ≡ 4 C – 5 CH
3
1-pentene-3-yne pent-1-ene-3-yne
If the double bond and the triple bond are at equal distance from the ends of the chain, number the chain so that the double bond receives a lower number than the triple bond
(because “ene” comes before “yne” in the alphabet).
1 CH
2
═ 2 CH – 3 CH
2
– 4 CH
2
– 5 C ≡ 6 CH
1-hexene-5-yne
Hex-1-ene-5-yne
EXAMPLES
Name the following alkene according to IUPAC system
3,4-dimethyl-1-pentyne
3,4-dimethyl-pent-1-yne
Number and name the substituents and write the name.
CH
3
– C ≡ C – CH – CH
3
|
CH
3
2-methyl-3-pentyne
2-methyl-pent-3-yne
Identify the structure. Give its IUPAC name.
4-ethyl-2-hexyne
4-ethylhex-2-yne
Draw the structure of 6-bromo-2-methylhept-3-yne
1 2 3 4 5 6 7
Draw the structure of pent-2-yne
1 2 3 4 5
6-chloro-4-ethyl-6-methyl-1,4-heptadiyne
6-chloro-4-ethyl-6-methyl-hepta-1,4-diyne
SYNTHESIS OF ALKYNES
1. Carbide process:
Acetylene is prepared on industrial scale by the reaction of calcium carbide (CaC
2
) with water.
CaC
2
+ H
2
O
CH ≡ CH + Ca(OH)
2
2. Dehydrohalogenation of vic – dihalide:
Alkynes can be prepared by the reaction of vic-
Dihalides with a strong base.
CH
2
Br – CH
2
Br NaOH CHBr═CH
– HBr
2
NaOH CH≡CH+ HBr
PHYSICAL PROPERTIES
OF ALKYNES
Physical State:
Alkynes with 1-4 Carbon atoms are gases.
Alkynes with 5-17 Carbon atoms are liquids.
Alkynes with more than 18 Carbon atoms are solids.
Melting & Boiling Point:
Alkynes usually have melting and boiling points greater than those of corresponding alkenes.
Solubility:
Alkynes being non – polar, are soluble in non-polar solvents such as CCl
4
, ether and benzene. They are generally insoluble in water.
Density:
The density of alkynes is generally somewhat higher than those of the corresponding alkenes.
Reactivity:
Since alkynes are also unsaturated hydrocarbons like alkenes, so their reactivity is more or less the same as that of alkenes.
REACTIONS OF ALKYNES
1. Hydrogenation:
Alkynes react with hydrogen to form alkenes or alkanes.
CH ≡ CH + H
2
Pt
CH
2
═CH
2
Pt/ H
2 CH
3
– CH
3
2. Halogenation:
Alkynes react with halogens to form di or tri haloalkanes depending upon the number of halogens added.
CH≡CH+Br
2
CCl4
CHBr ═CHBr
Br
2 /
CCl4
CHBr
2
– CHBr
2
25oC 25oC
3. Hydrohalogenation:
Alkynes can react with hydrogen halides to form either haloalkanes or gem-dihaloalkanes(compound in which halogens are attached to the same carbon) depending upon the number of hydrogen halides added.
CH≡CH + HBr
CH
2
═CHBr+HBr
CH
3
– CH – Br
2
4. Hydration:
Alkynes when react with water , they give aldehydes or ketones. Only acetylene gives aldehyde while all other alkynes give ketone. O
║
CH≡CH+ H
2
O H
2
SO
4
CH
2
═CH – OH
O
CH
3
–C–OH
RC≡CH+H
2
O H
2
SO
4
RC ═CH
2
R – C – CH
3
Uses
Acetylene, the simplest alkyne, is used in torches to generate temperatures of around 3,000 degrees Celsius to weld metal by generating oxy-acetylene welding.
It is also used as a starting material in the synthesis processes used to make polymers, and as a solvent.
Alkynes are generally used as the starting materials for the manufacture of a large number of organic compounds of industrial importance such as, chloroprene, vinyl chloride etc.
Alkynes are also highly important parts of some antitumor agents
.