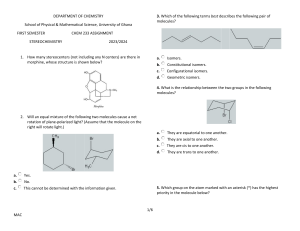
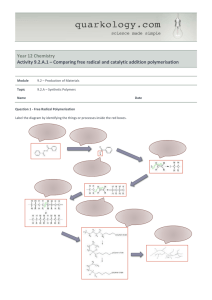
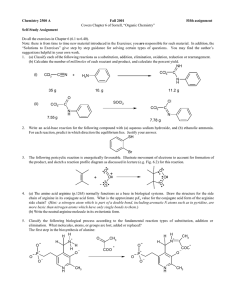
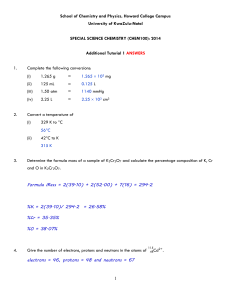
امتحان فصلى كيمياء عضوية 1
advertisement

,vghn hnMajmaah University Science, Zulfi College of First midterm exam ( CHEM109) Medical Lab. student Student name………………………….. ID:……………………… ------------------------------------------------------------------------------------------------------I. Choose the correct answer: 1. Which type of molecule contains double bonds? a. Alkane b. alkene c. alkyne 2. The general formula for the alkynes is a. CnH2n+2 b. CnH2n-2 c. CnH2n d. CnH2n+1 3. Which is the appropriate conversion of CH3CHClCH2CH(CH3)2 to a skeletal line formula? 4. In a molecule of C3H6, the total number of covalent bonds is a. 10 b. 11 c. 9 5. How are the molecules in the following pair related? a. they are two isomers. b. they are resonance structures. C. neither of the choices is correct. 6. What is the hybridization of the indicated atoms in the following compound? 1 7. Which of the following statements is (are) correct? a.Ethene has sp3 carbon atoms and the geometry around each carbon is trigonal planar. b.Ethene has sp3 carbon atoms and the geometry around each carbon is tetrahedral. c.Ethene has sp2 carbon atoms and the geometry around each carbon is trigonal planar. 8. The shape of the ethane molecule is a.planer b. linear c. tetrahedral 9. The halogenation of methane, is an example of _________. a. Electrophilic addition reaction radical substitution reaction b. elimination reaction c. free 10. which of the following is the most acidic a. propane b. propene d. propyne II. Answer the following question: 1. Write the IUPAC name of the following compound CH3 CH3 CH2 CH CH C CH3 CH3 2. Draw the molecular structure of the following compound '' 3. 2-methylbutane '' Draw two different isomers of the following molecular formula C4H10 2 III.Complete the following equations: Best wishes 3