Chapter Four Ouline Part Two
advertisement

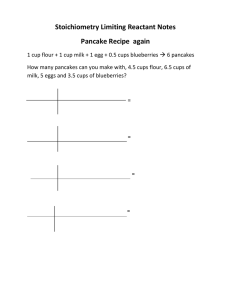
AP Chapter Four Outline Part Two I. The Mole and Chemical Reactions In a balanced chemical equation, the coefficients on each species can be interpreted as the numbers of moles of each compound. These coefficients also provide the mole ration that relates the numbers of moles and reactants and products to each other. Example: CH4 + 2O2 → CO2 + 2H2O One mole of methane reacts with two moles of oxygen to form one mole of carbon dioxide and two moles of water II. Reactions with One Reactant in Limited Supply A. Limiting Reactant: the reactant that is completely converted to products during a reaction. The limiting reactant must be used as the basis for calculating the maximum amount of product. 1. The moles of product formed are always determined by the starting number of moles of the limiting reactant. III. Percent Yield A. Theoretical Yield: the maximum possible quantity of product in a chemical reaction. B. Actual Yield: the quantity of desired product actually attained. C. Percent Yield: (actual yield / theoretical yield) x 100%