CHAPTER_6_PbsaRESOUDRE.doc
advertisement
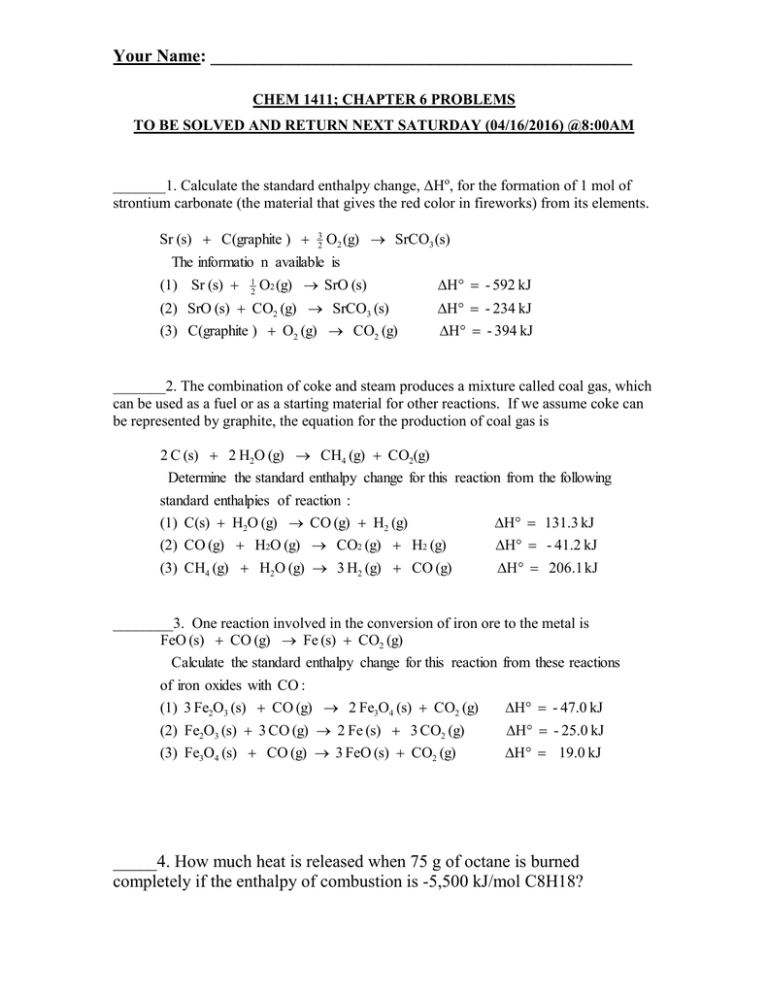
Your Name: ________________________________________________ CHEM 1411; CHAPTER 6 PROBLEMS TO BE SOLVED AND RETURN NEXT SATURDAY (04/16/2016) @8:00AM _______1. Calculate the standard enthalpy change, ΔHo, for the formation of 1 mol of strontium carbonate (the material that gives the red color in fireworks) from its elements. Sr (s) C(graphite ) 3 2 O2 (g) SrCO3 (s) The informatio n available is (1) Sr (s) 1 2 O2 (g) SrO (s) H - 592 kJ (2) SrO (s) CO2 (g) SrCO3 (s) H - 234 kJ (3) C(graphite ) O2 (g) CO2 (g) H - 394 kJ _______2. The combination of coke and steam produces a mixture called coal gas, which can be used as a fuel or as a starting material for other reactions. If we assume coke can be represented by graphite, the equation for the production of coal gas is 2 C (s) 2 H2O (g) CH4 (g) CO2(g) Determine the standard enthalpy change for this reaction from the following standard enthalpies of reaction : (1) C(s) H2O (g) CO (g) H2 (g) H 131.3 kJ (2) CO (g) H2O (g) CO2 (g) H2 (g) (3) CH4 (g) H2O (g) 3 H2 (g) CO (g) H - 41.2 kJ H 206.1 kJ ________3. One reaction involved in the conversion of iron ore to the metal is FeO (s) CO (g) Fe (s) CO2 (g) Calculate the standard enthalpy change for this reaction from these reactions of iron oxides with CO : (1) 3 Fe2O3 (s) CO (g) 2 Fe3O4 (s) CO2 (g) H - 47.0 kJ (2) Fe2O3 (s) 3 CO (g) 2 Fe (s) 3 CO2 (g) H - 25.0 kJ (3) Fe3O4 (s) CO (g) 3 FeO (s) CO2 (g) H 19.0 kJ _____4. How much heat is released when 75 g of octane is burned completely if the enthalpy of combustion is -5,500 kJ/mol C8H18? Your Name: ________________________________________________ C8H18 + 25/2 O2 → 8CO2 + 9H2O a. 7200 kJ b. 8360 kJ c. 4.1 × 105 kJ d. 3600 kJ e. 5500 kJ ______5. Calculate the amount of heat released in the complete combustion of 8.17 grams of Al to form Al2O3(s) at 25°C and 1 atm. ΔH for Al2O3(s) = 1676 kJ/mol 4Al(s) + 3O2(g) → 2Al2O3(s) a. 254 kJ b. 203 kJ c. 127 kJ d. 237 kJ e. 101 kJ ______6. Given the following at 25°C and 1.00 atm: ΔH0 1/2N2(g) + O2(g) → NO2(g) 33.2 kJ N2(g) + 2O2(g) → N2O4(g) 11.1 kJ Calculate the ΔH0 for the reaction below at 25°C. 2NO2(g) → N2O4(g) a. +11.0 kJ b. +44.3 kJ c. +55.3 kJ d. -22.1 kJ e. -55.3 kJ ______7. Calculate ΔH0 for the following reaction at 25.0C. Fe3O4(s) + CO(g) → 3FeO(s) + CO2(g) ΔH (kJ/mol) -1118 -110.5 -272 -393.5 a. -263 kJ b. 54 kJ c. 19 kJ d. -50 KJ e. 109 kJ Your Name: ________________________________________________ ______8. How much heat is released when 6.38 grams of Ag(s) reacts by the equation shown below at standard state conditions? 4Ag(s) + 2H2S(g) + O2(g) → 2Ag2S(s) + 2H2O(l) Substance Ag(s) 0 H2S(g) -20.6 O2(g) 0 Ag2S(s) -32.6 H2O(l) -285.8 a. 8.80 kJ b. 69.9 kJ c. 22.1 kJ d. 90.8 kJ e. 40.5 kJ _____9. Which of the following is not a state function? a. temperature b. pressure c. work d. volume e. enthalpy Your Name: ________________________________________________ Use additional paper if it’s necessary to show all your work for full credit 1. Given the following equations and Ho values, determine the heat of reaction (kJ) at 298 K for the reaction: B2H6(g) + 6 Cl2(g) 2 BCl3(g) + 6 HCl(g) BCl3(g) + 3 H2O(l) H3BO3(g) + 3 HCl(g) Ho/kJ = -112.5 B2H6(g) + 6 H2O(l) 2 H3BO3(s) + 6 H2(g) 1/2 H2(g) + 1/2 Cl2(g) HCl(g) Ho/kJ = -493.4 Ho/kJ = -92.3 Your Name: ________________________________________________ 2. Given the following equations and Ho values, determine the heat of reaction (kJ) at 298 K for the reaction: 2 OF2(g) + 2 S(s) O2(g) + 2 HF(g) Ho/kJ = -276.6 OF2(g) + H2O(l) SF4(g) + 2 H2O(l) S(s) + O2(g) SO2(g) + SF4(g) 4 HF(g) + SO2(g) Ho/kJ = -827.5 SO2(g) Ho/kJ = -296.9 3. Determine Ho/kJ for the following reaction using the listed enthalpies of reaction: N2H4(l) + 2 H2O2(g) N2(g) + 2 H2O(l) Ho/kJ = -622.3 kJ N2H4(l) + O2(g) H2(g) + 1/2 O2(g) H2(g) + O2(g) N2(g) + 4 H2O(l) H2O(l) H2O2(l) Ho/kJ = -285.8 kJ Ho/kJ = -187.8 kJ Your Name: ________________________________________________ 4. Calculate the value of Ho/kJ for the following reaction using the listed thermochemical equations: P4O10(g) + 6 PCl5(g) 1/4 P4(s) + 3/2 Cl2(g) P4(s) + 5 O2(g) PCl3(g) P4O10(g) PCl3(g) + Cl2(g) PCl3(g) + 1/2 O2(g) 10 Cl3PO(g) PCl5(g) Ho/kJ = -306.4 Ho/kJ = -2967.3 Ho/kJ = -84.2 Cl3PO(g) Ho/kJ = -285.7 5. Given the following equations and Ho values, determine the heat of reaction (kJ) at 298 K for the reaction: 2 SO2(g) + 2 P(s) + 5 Cl2(g) 2 SOCl2(l) + 2 POCl3(l) SOCl2(l) + H2O(l) SO2(g) + 2 HCl(g) Ho/kJ =+10.3 PCl3(l) + 1/2 O2(g) POCl3(l) Ho/kJ = -325.7 P(s) + 3/2 Cl2(g) PCl3(l) Ho/kJ = -306.7 4 HCl(g) + O2(g) 2 Cl2(g)+2 H2O(l) Ho/kJ = -202.6 Your Name: ________________________________________________ 6. Calculate the heat of combustion (kJ) of propane, C3H8 using the listed standard enthapy of reaction data: C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) 3 C(s) + 4 H2(g) C3H8(g) C(s) + O2(g) CO2(g) H2(g) + 1/2 O2(g) H2O(g) Ho/kJ = -103.8 Ho/kJ = -393.5 Ho/kJ = -241.8 7. Given the following equations and Ho values given below, determine the heat of reaction at 298 K for the reaction: 2 N2(g) + 5 O2(g) 2 N2O5(g) 2 H2(g) + O2(g) 2 H2O(l) Ho/kJ = -571.6 N2O5(g) + H2O(l) 2 HNO3(l) Ho/kJ = -73.7 N2(g) + 3 O2(g) + H2(g) 2 HNO3(l) Ho/kJ = -348.2 Your Name: ________________________________________________ 8. Given the following equations and Ho values, determine the heat of reaction at 298 K for the reaction: C(s) + 2 H2(g) CH4(g) C(s) + O2(g) CO2(g) Ho/kJ = -393.5 H2(g) + 1/2 O2(g) H2O(l) Ho/kJ = -285.8 CO2(g) + 2 H2O(l) CH4(g) + 2 O2(g) Ho/kJ = +890.3






