thermodynamics, energy, heat engines

First law of thermodynamics first law of thermodynamics:
heat added to a system goes into the internal energy of the system and/or into doing work heat in = work + change in internal energy:
Q = W + U is different formulation of energy conservation for isolated system:
no heat flow if work done, must reduce internal energy historical note:
1st law quoted as a law in its own right because it took a long time to realize that heat is a form of energy. Until around 1800, heat was considered a fluid called “caloric” that is contained in materials, can be soaked up by materials,..
It took about 50 years to replace this with the new paradigm that heat is a form of energy, and that total energy, including thermal energy, is conserved.
Milestones on path to first law:
Experiments and observations by Benjamin
Thompson, James Prescott Joule, Julius Robert
Mayer,.. and conjectures by Mayer, Hermann
Helmholtz, Rudolf Clausius,..
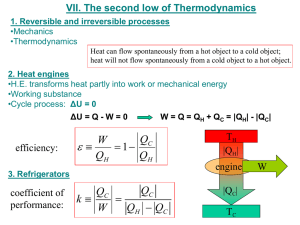
HEAT ENGINES
heat engine:
is a device that converts heat into work principle: heat input, some of it used to do work, some of it discarded operate in cyclical process, i.e. at end of an “engine cycle”, engine must be in same state as before; for a cyclical process: U net
= 0, Q = W, i.e. work done = net heat input = (heat in) - (heat out) heat engine operates between two “reservoirs”; reservoir = system from which heat may be readily extracted and into which heat can be deposited at given temperature; heat engine takes heat from high temperature reservoir, converts some of it into work, and ejects rest of heat into low temperature reservoir; example: car engine:
hot reservoir = cylinder in which air-fuel mixture is exploded; cold reservoir = environment to which waste heat is expelled; thermal efficiency of a heat engine = ratio of work output to heat input:
= W/Q in
= W/ Q h
= 1 - (Q c
/Q h
)
heat engines and refrigerators
engine
refrigerator
CARNOT ENGINE
Nicolas Léonard Sadi Carnot (1796 -1832)
(“Réflexions sur la puissance motive de la chaleur”,
1824) constructed idealized method for extracting work with greatest possible efficiency from an engine with heat-flow from one substance at higher temperature to another substance at lower temperature, - the “Carnot cycle”
Carnot cycle is reversible process - can run in either direction;
Carnot engine:
container with piston
can be brought into thermal equilibrium with two heat reservoirs, one at high temperature
T h
, one at low temperature T c
; or can be isolated from outside world (i.e. no heat-flow to or from container);
isothermal process: temperature constant; adiabatic process: isolated no heat exchange; efficiency of Carnot engine:
= 1 - (T c
/T h
)
(note temperature here is measured in Kelvin)
Carnot engine is the most efficient engine possible (2nd law of thermodynamics).
Second law of thermodynamics
several different formulations of 2nd law;
all can be shown to be equivalent:
law of heat flow: “Heat (thermal energy) flows spontaneously (i.e. without external help) from region of higher temperature to region of lower temperature. By itself, heat will not flow from cold to hot body.
Kelvin formulation: No process is possible whose sole result is the removal of heat from a source and its complete transformation into work.
Clausius formulation: No process is possible whose sole result is the transfer of thermal energy from a body at low temperature to a body at high temperature. heat engine formulation: No heat engine can be more efficient than the Carnot engine.
consequence of 2nd law:
the quality of thermal energy (its ability to do work) depends on the temperature; thermal energy at low temperature less useful than thermal energy at high temperature;
“using energy” does not mean destroying it (cannot be destroyed); it means converting it into work and thermal energy at lower temperature than before
”degradation of energy”
ENTROPY
Entropy:
when heat Q at temperature T enters a system, the system's entropy S changes by
S = Q/T for the Carnot cycle: Q temperature T temperature T c h
, Q
c h taken from reservoir at given to reservoir at
S = Q h
/T h
- Q c
/T c the change in entropy is = 0.
= 0, i.e. for the Carnot cycle, for other cyclical processes: 2nd law of thermodynamics efficiency smaller than that of
Carnot process
entropy formulation of 2nd law of thermodynamics:
For any process, the total entropy of all the participants either increases or stays the same; it cannot decrease.
entropy related to the degree of disorder, to the probability of a state; order is less probable than disorder (there are many more ways of having disorder than there are of having order); some systems (e.g. living things and beings) decrease their entropy, but at the cost of increasing the entropy of the rest of the universe.