SCI 111
advertisement

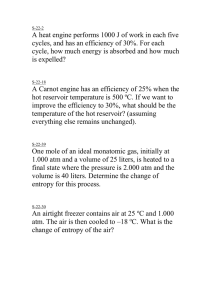
SCI 111 HOMEWORK 3 Thermodynamics 1. Estimate the number of water molecules in a human body with mass 80 kg. The water is ~62% of all the mass of a human. 2. What is the mass density of hydrogen at pressure P=1 atm and temperature T=30o C? 3. Verify that the units for PV (pressure times volume) are units for energy, i.e., joules. 4. What is the temperature of an ideal gas whose molecules have an average translational kinetic energy 5 x 10-19 J? 5. What is the total internal energy of 3 moles of an ideal gas at temperature 25o C ? 6. A cylinder contains 250 liters of hydrogen gas at 0o C and pressure 10 atm. How much energy is needed to raise the temperature to 300 C? 7. State the Second Law of Thermodynamics in its 3 different forms. 8. Can you have an engine taking 600 J of heat at T=1000 K, releasing 200 J of heat at 300 K and doing 400 J of work? 9. Can you have a reversible engine which works between two heat reservoirs – one at temperature 900 K, the other at temperature 450 K - such that for each 250 kJ of heat taken from the hot reservoir the engine does 110 kJ of work? Explain. 10. A heat engine takes in 125 kJ of heat from a reservoir at 815 K and exhausts 82 kJ to a reservoir at 293 K. What is the efficiency of this engine? If you consider the two reservoirs and the engine as a closed system, by how much does the entropy change? Consider now an ideal engine working between these two reservoirs. What would its efficiency be. By how much would entropy change in this case? 11. The outside temperature is -100C (minus ten), the temperature in the room is 200 C. Heat flows from the room at a rate of 200 J each second. What is the change of the entropy of the universe each second? 12. If the number of microstates for each particle of a thermodynamic system triples, how much does the entropy increase? (The system has N particles.) 13. You are throwing 2 dices. Your “macrostate” is the sum of the numbers shown on the faces that are up. The initial state is 11. You shake the dices and throw them and the final state is 6. By how much has the entropy changed?