VII. The second low of Thermodynamics 1. Reversible and irreversible processes •Mechanics •Thermodynamics
advertisement
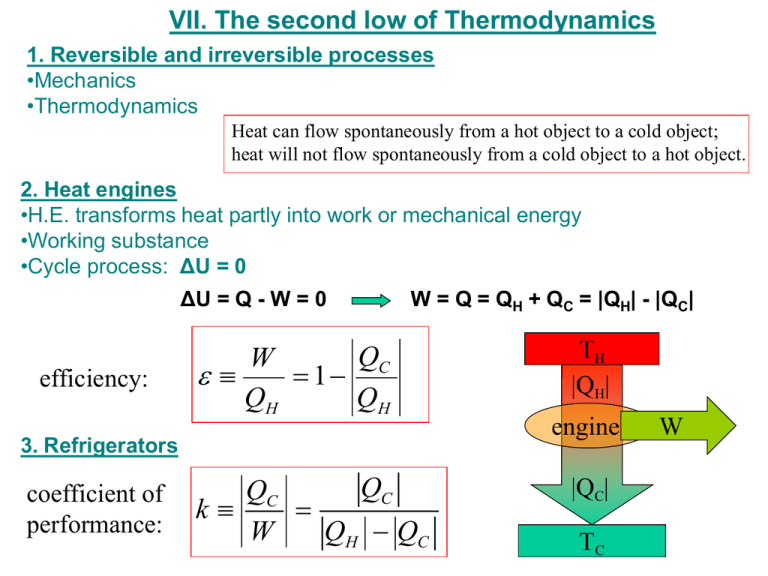
VII. The second low of Thermodynamics 1. Reversible and irreversible processes •Mechanics •Thermodynamics Heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. 2. Heat engines •H.E. transforms heat partly into work or mechanical energy •Working substance •Cycle process: ΔU = 0 ΔU = Q - W = 0 efficiency: W = Q = QH + QC = |QH| - |QC| QC W 1 QH QH 3. Refrigerators coefficient of performance: QC QC k W QH QC TH |QH| engine |QC| TC W 4. The second law of thermodynamics and heat engines It is impossible to build a heat engine with 100% efficiency 5. The Carnot Cycle (reversible) QC TC QH TH a P Maximum efficiency: TC TH TC C 1 TH TH b TH d c TL TC kC TH TC V 6. The Carnot Cycle and the second law of thermodynamics No device is possible whose sole effect is to transform a given amount of heat completely into work No device is possible whose sole effect is to transform heat from one system at temperature TL into a second system at higher temperature TH Example 1: A nuclear power plant generate steam at 6300 C. The steam is sent through a series of turbines and exits at 1000 C. What is the maximum possible efficiency of the power station? TH = 630ºC TC = 100ºC εC - ? TH TC C TH 630 C 630 C 273.15 903.15K 100 C 100 C 273.15 373.15K (630 100) K C 0.59 903.15K C 59% Example 2: An ice-making machine operates in a Carnot cycle. It takes heat from water at 0.0C and rejects heat to a room at 24.0C. Suppose that 50.0 kg of water at 0.0C are converted to ice at 0.0C. How much energy must be supplied to the device? Heat of fusion of water is 334*103 J/kg. Q mL TH = 24.0C TC = 0.0C QC TC kC W TH TC m = 50.0 kg L = 334*103 J/kg W-? TH TC TH TC W QC mL TC TC (24.0 0.0) K W 50.0kg 334 10 J / kg 1.47 106 J 273.15K 3 Question In one cycle, a heat engine does 10 J of work and exhausts 20 J of waste heat. The thermal efficiency of the heat engine is ___ %. 1. 2. 3. 4. 0 20 33 50 W QH QC W W 10 J 0.33 QH W QC 10 J 20 J 33% Question In a Carnot heat engine, the cold temperature reservoir is at 300 K (= 27 ˚C) and the thermal efficiency is 50%. 1. 2. 3. 4. The temperature of the hot reservoir is ___ K. TC C 1 TH TC 1 C TH 400 600 800 1000 TH TC 1 C TH 300K 1 0.5 600K Question A refrigerator has a coefficient of performance K = 2.00. Work is done on the refrigerator at a rate Pwork = dW/dt = 100 Watts. 1. 2. 3. 4. The rate at which heat is removed from inside the refrigerator is d|QC|/dt = ___ Watts. QC k W QC k W d QC dt k dW d QC dt dt 200 Watts 50 100 200 400 The internal-combustion engine • A fuel vapor can be compressed, then detonated to rebound the cylinder, doing useful work. 6. Otto cycle QC W 1 QH QH Otto 1 1 r 1 r - compression ratio γ – CP/CV Example: An automobile engine has a compression ratio of 10.0. The maximum theoretical thermal efficiency of the engine is ___ %. ( = 1.40 for air) Otto 1 1 10 1.4 1 0.60 60%