Thermodynamics
advertisement

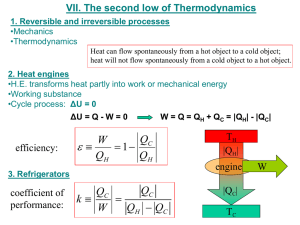
Thermodynamics CHAPTER 15 15.1 Thermodynamic Systems and Their Surroundings Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. The work done on an object (or system) is equal to the change in kinetic energy A system is the collection of objects being studied or analyzed. Diathermal walls permit heat to flow through the surroundings of a system. Adiabatic walls are perfectly insulated and would therefore not permit heat to flow between the systema nd the surroundings. 15.2 The Zeroth Law of Thermodynamics Two systems individually in thermal equilibrium with a third system are in thermal equilibrium with one another. Thermal equilibrium is established when no net flow of heat exists between systems. Temperature is the indicator of thermal equilibrium. 15.3 The First Law of Thermodynamics Another way of stating the Law of Conservation of Energy. When a system gains heat, the internal energy of the system increases. Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negative when it is done on the system. 1st Law Summarized The internal energy of a system changes from an initial value Ui to a final value Uf due to heat Q and work W. Q is positive when the system gains heat and negative when it loses heat. W is positive when work is done by the system and negative when work is don on the system. Read Example 1 and 2 in text! U U f U i Q W U, Q, W ALL Types of Energy (JOULES) U • Internal energy • Depends only on the state of a system Q • Heat energy • Dealt with in Chapters 12-14 W • Work • A change in kinetic energy due to forces acting over a distance. 15.4 Thermal Processes Quasi-Static – the process whereby heat and work interact with surroundings. The process occurs slowly enough that a uniform pressure and temperature exist throughout all regions of the system. Isobaric process occurs at constant pressure. Positive value for work done by a system when it expands isobarically. Negative value for work when work is done one the system to compress it. W PV P(V f Vi ) More Thermal Processes Isochoric process – occurs at constant volume Heat will only serve to change the internal energy. Isothermal process – occurs at constant temperature Adiabatic process – occurs without the transfer of heat Internal energy will decrease by exactly the amount of work done when work is done by a system. Internal energy will increase when work is done on a system. 15.5 Thermal Process Using an Ideal Gas Isothermic Expansion or Compression occurs when a contained gas expands or contracts due to a slowly increasing or decreasing force. W nRT ln( Vf Vi ) PiVi Pf V f 15.6 Specific Heat Capacities Instead of using mass to solve for specific heat, number of moles is often a helpful method. Letter C refers to the molar specific heat capacity. Use Kelvin as the unit for temperature. Cp and Cv must be used depending on constant pressure or volume conditions. Q CnT The difference in C values exists because work is done when the gas expands in response to the addition of heat under constant pressure. No work is done under constant volume. 5.7 The 2nd Law of Thermodynamics Heat flows spontaneously from a substance of higher temperature to a substance at a lower temperature and does not flow spontaneously in the reverse direction. 15.8 Heat Engines Any device that uses heat to perform work. Three features Q is supplied to the engine at a high input temperature from a hot reservoir. Part of the input Q us used to do work by the working substance of the engine, which is the material within the engine actually doing the work. The remaining input Q is rejects to a place called a cold reservoir, which has a temperature lower than the input temperature. No negative values will exist for Q for W Efficiency The ratio of the magnitude of the work done by the engine to the magnitude of the input heat. Often expressed as a % In reality, can never by 100% e 1 Qc QH Carnot’s Principle and the Carnot Engine An alternative statement for the 2nd Law of Thermodynamics No irreversible engine operating between two reservoirs at constant temperatures can have a greater efficiency than a reversible engine operating between the same temperatures. Furthermore, all reversible engines operating between the same temperatures have the same efficiency. Reversible process: both the system and its environment can be returned to exactly the states they were in before the process occurred. Carnot Engine A reversible engine in which all input heat originates from a hot reservoir at a single temperature and all rejected heat goes into a cold reservoir at a single temperature. TC e 1 TH 15.10 Refrigerators, Air Conditioners, and Heat Pumps Fridge – takes heat from inside and deposits outside Air Conditioner – room is cold reservoir and outdoors is hot Heat Pump – makes heat from cold outdoors into a warm house These devices MAKE energy flow against the natural tendency of hot to cold! 15.11 Entropy A function of the state or condition of a system Can be defined as the partial loss of efficiency in a machine due to the inability of some energy to do work. Reversible processes do not alter the total entropy of the universe. Any irreversible process increases the entropy of the universe. In terms of the 2nd Law : the total entropy of the universe does not change when a reversible process occurs and increases when an irreversible process occurs. Order and Disorder Heat flow into a system increases the disorder of a system according to the equation Systems naturally move toward a more disordered state Q S T R 15.12 The 3rd Law of Thermodynamics It is NOT possible to lower the temperature of any system to absolute zero (T = 0 Kelvin) in a finite number of steps.