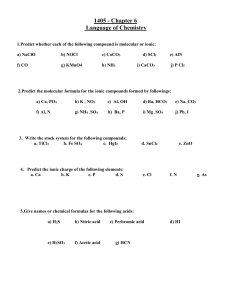
Chemical Names & Formulas Water Ammonia Methane 1 Why “Systematic Names” ? # atomic particles 3 (p, n, e) # elements 110+ # elements in 8 earth’s crust (99%) # elements in all 25 living things # compounds >14,000,000 2 Why “Systematic Names” ? Water Lime Lye Potash Table Salt Laughing Gas Baking Soda H2O CaO NaOH K2CO3 NaCl N2O NaHCO3 3 Atoms and Ions Atom: neutral no net charge + (# p = # e ) e.g. Na 11 and 11 e + Cl 17 p and 17 e + p 4 Atoms and Ions e e e e Ion: e atom (or group of atoms) has + or – charge has more or less e only the # of e changes + + e.g. Na 11 p and 10 e + Cl 17 p and 18 e 5 Atoms and Ions Na vs. + Na Very different and Cl vs. Cl Demo: Fe vs. +3 Fe 6 Ions Positive charge: “cation” + Na 2+ Ca 3+ Al These atoms lost electrons Negative charge: “anion” Cl 2 O SO4 2- These atoms gained electrons 7 Compounds Substances composed of 2 or more different atoms HCl NaCl CO2 8 Chemical Formulas Shows the kinds and numbers of each type of atom in a chemical compound. Water = H2O two atoms of H one atom of O 9 Compounds Molecular •Atoms bonded as a unit •nonmetals e.g. H2O Ionic •Ions held together by + and - charges •metal + nonmetal e.g. NaCl 10 Charges NOT shown Molecular & Ionic Compounds 11 1. Molecular Formulas Molecules are represented by molecular formulas: discrete units of bonded nonmetals. e.g H2O & CO2 O H H “covalent” bond “structural” formula for water 12 Naming Molecular Compounds (bonded nonmetal atoms) st nd prefix-1 atom-prefix-2 atom-ide number N2O5 = dinitrogen pentoxide 13 Number 1 2 3 4 5 6 7 8 9 10 Prefix Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Note: don’t use ‘mono’ for the first atom. 14 Try It CO2 N2 O PCl3 SF6 SO3 N2O4 H2O Dangerous chemical 15 Ionic Formulas Ionic compound is represented by a formula unit, the lowest ratio of atoms in the compound. NaCl “ionic” bond + Na and Cl 16 Naming Ionic Compounds (positive metal ion + negative nonmetal ion) First must learn ion names and charges! 17 Monatomic Ions (single atom ions) Metals form cations (+) +2 Mg loses two e to form Mg Nonmetals form anions (-) The name ends in “ide” Cl gains one e to form Cl Charge from Periodic Table 18 Common Monatomic Ions +1 +2 +3 1 2 + Li 2+ Be + Na 2+ Mg + K 2+ Ca + Rb 2+ Ba 13 3+ Al -3 -2 -1 14 15 16 17 18 3N 2O F 3P S 2- Cl 3As 2Se Br I end in --ide 19 Monatomic Ions: Try It!!! When the following elements become ions, state: ion name charge number of e lost or gained Ca S P Br K Al 20 Monatomic Ions: Some metals can form two different types of ions. Iron: +2 Fe Copper: +1 Cu and +3 Fe and +2 Cu •See Periodic Table for charges. 21 Multiple Charges: Names •Stock System: “atom (charge)” 2+ Fe is Iron(II) ion 3+ Fe is Iron(III) ion 22 Multiple Charges: Names •Classical System: Latin name ending in “ous” for lower charge “ic” for the higher charge 2+ Fe is Ferrous ion 3+ Fe is Ferric ion 23 Others Ion + Cu 2+ Cu 2+ Pb 4+ Pb 2+ Sn 4+ Sn Stock Copper(I) Copper(II) Lead(II) Lead(IV) Tin(II) Tin(IV) Classic Cuprous Cupric Plumbous Plumbic Stannous Stannic 24 Polyatomic Ions Some atoms group together as a unit to form an ion. NO3 - 25 Polyatomic Ion: Charged group of atoms acting as a unit NO3 nitrate ion SO4 PO4 3- phosphate ion 2- + NH4 sulfate ion ammonium ion 26 Regents Table ‘E’ Note “ite” & “ate” pairs --ite --ate 2- sulfite: SO3 nitrite: NO2 chlorite: ClO2 2- sulfate: SO4 nitrate: NO3 chlorate: ClO3 Be able to recognize them !!! 27 Ionic Formulas Name: cation then anion name •Potassium chloride + K Cl KCl Charges must “balance” But don’t write charges! 28 Ionic Formulas •Calcium bromide 2+ Ca Br CaBr2 (balance charge) •Copper(I) sulfate + 2Cu SO4 Cu2SO4 29 Shortcut “Criss Cross” Copper(I) sulfate + Cu 2SO4 Cu2(SO4)1 = Cu2SO4 Need to recognize polyatomic ions 30 Shortcut “Criss Cross” Calcium sulfide 2+ 2Ca S Ca2S2 = CaS (reduce) 31 Shortcut “Criss Cross” Iron(III) Carbonate 3+ 2Fe CO3 Fe2(CO3)3 (brackets needed for polyatomic ion with subscript) 32 Name or Give the Formula of That Compound!!! Silver chloride CuBr2 Ammonium bromide Mg3(PO4)2 Calcium chromate 33 Try Some More!! N2O5 PCl3 AlCl3 Sodium hydrogen sulfate SnO2 34 General Properties Molecular Compounds Weak “intermolecular forces” (molecular attractions) Low melting & boiling points Ionic Compounds Strong ionic attractions High melting & boiling points 35 Naming Acids •Acids are a special class of + compounds with H as the cation. Example: + H with Cl HCl(aq) where (aq) = dissolved in water 36 Naming Acids Acids are named according to the anion. 1. If the anion ends in –ide, the acid is hydro----ic acid. Cl is chloride HCl(aq) is hydrochloric acid 37 Naming Acids 2. If the anion ends in –ite, the acid is ----ous acid. 2 SO3 is sulfite H2SO3(aq) is sulfurous acid 38 Naming Acids 3. If the anion ends in –ate, the acid is –ic acid. NO3 is nitrate HNO3(aq) is nitric acid 39 Naming Acids: Try It Name HCN(aq) HClO4(aq) HClO(aq) HCl(aq) 40 Naming Acids: Try It Write the formula for: carbonic acid nitrous acid Regents Table K 41 Summary: Names & Formulas •Atoms vs. ions + Na Na •Compounds: -molecular (nonmetals) -ionic (metal + nonmetal) cation(+) anion(-) 42 Summary: Names & Formulas •Molecular compound: -prefix-atom-prefix-atom-ide N2O5 = dinitrogen pentoxide 43 Summary: Ionic compounds -know names & charges •monatomic ions (PeriodicTable) •transition metals -stock system: iron(II) •polyatomic ions: NO3 •compound name: cation+anion 44 45 Warm-up How many p+ and e- in Ca and Ca+2? Is Ca+2 and anion or cation? What are the two general types of compounds? How do you recognize each? 46 Warm-up Name NO2 and N2F4 47 Warm-up Give the symbol and the name of the ion that each element forms: Mg Al P Br How do you know NH4NO3 is ionic even though it has only nonmetal atoms? 48 Warm-up 1. Fill in the table: Molecular Ionic Types of atoms Name of formula Name of bond 2. Name N2O and Al2(SO4)3. 49 Name or give formula: gold(I) sulfate S2Cl5 barium phosphide Fe3(PO4)2 calcium hydrogen carbonate 50 Warm-up Write the formula unit for sodium sulfate. Why can’t this formula be reduced? 51 Name or give formula: NH4+ S-2 SO3-2 NaCN SnO PBr3 SO3 OH- magnesium nitride calcium sulfate aluminum hydrogen sulfate barium phosphate FeC2O4 N2O Au2SO4 52 Warm-up Name or write formula: •H2SO3 •Hydroiodic acid •N2S4 •FeSO3 53 Warm-up Name or write formula: •lead(IV) carbonate •barium nitride •CO •SnS2 •sulfurous acid 54