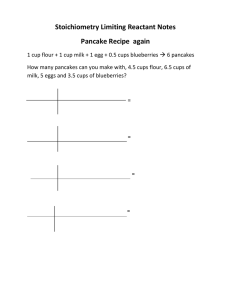
Practice Problem 1405 chapter 9.doc
advertisement

CHEM 1405 - CHAPTER 9 CHEMICAL EQUATION CALCULATIONS PRACTICE PROBLEMS 1. Find the mole, molecule, mass, and volume relationship in the following equation 4NH3 + 5O2 4NO + 6H2O 2. The complete combustion of octane, C8H18, a component of gasoline, proceeds as Follows: 2 C8H18 + 25 O2 16 CO2 + 18 H2O a) How many moles of CO2 are produced when 1.50-mol octane reacted? b) How many grams water produced in this reaction? c) How many moles oxygen required forming 90.0 g water? 3. In a certain experiment 2.50 g of NH3 reacts with 2.85 g of O2. Which reactant is the limiting reactant? How many grams of NO are formed? How much excess reactant remains after the liming reactant is completely consumed? 4. Given 30.0 g benzene and 65.0 g bromine in the following reaction: C6H6 + Br2 C6H5Br + HBr If the actual yield of C6H5Br, bromobenzene, is 56.7 g, what is the percentage yield?