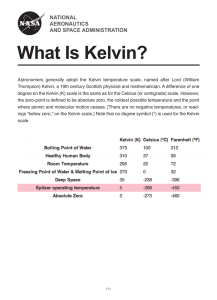
TAP 601- 8: Changing pressure and volume by changing temperature
advertisement
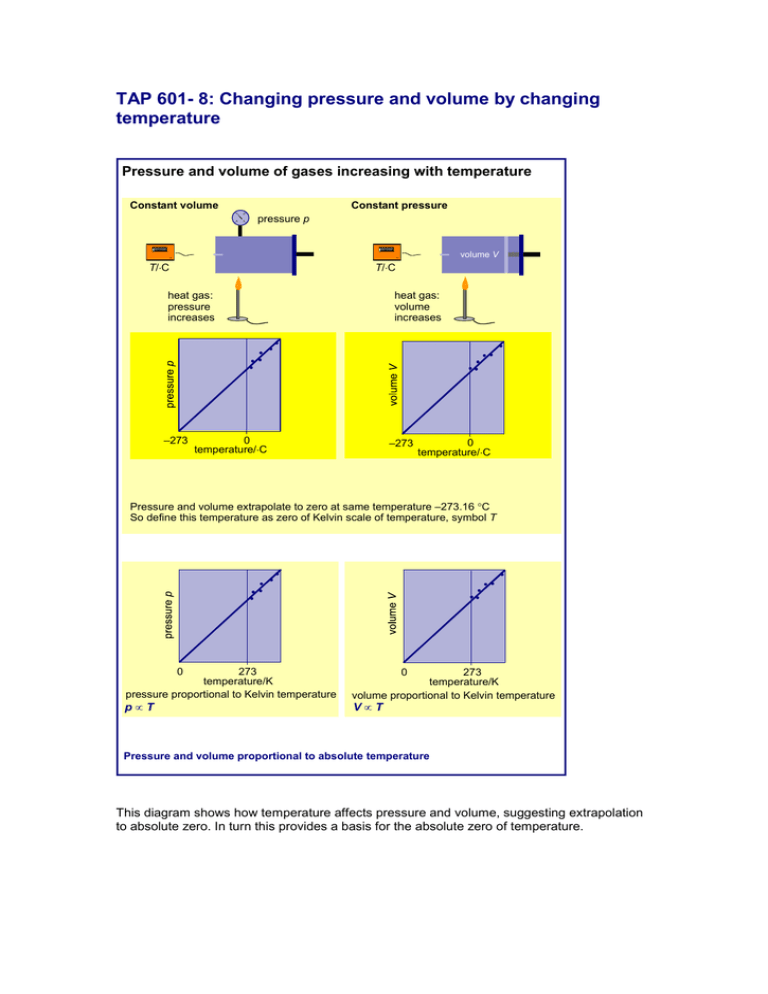
TAP 601- 8: Changing pressure and volume by changing temperature Pressure and volume of gases increasing with temperature Constant volume Constant pressure 1 2 4 3 pressure p 45.1 45.1 volume V T/C T/C heat gas: pressure increases –273 heat gas: volume increases 0 temperature/C –273 0 temperature/C Pressure and volume extrapolate to zero at same temperature –273.16 C So define this temperature as zero of Kelvin scale of temperature, symbol T 0 273 temperature/K pressure proportional to Kelvin temperature 273 temperature/K volume proportional to Kelvin temperature pT VT 0 Pressure and volume proportional to absolute temperature This diagram shows how temperature affects pressure and volume, suggesting extrapolation to absolute zero. In turn this provides a basis for the absolute zero of temperature. Practical advice These diagrams are reproduced here so that you can discuss them with your class. External reference This activity is taken from Advancing Physics chapter 13, 20O