Thermal Physics 3 - Gas Laws
advertisement

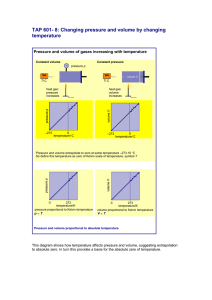
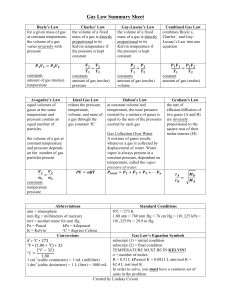
Gas Laws & Absolute Zero Thermal Physics Lesson 3 Learning Objectives State the three gas laws, describing the relationships between p,V,T and mass. Describe how the thermodynamic scale is defined. Define absolute zero. Convert temperatures between Celsius and Kelvin. Thermodynamic Temperature Scale A change of 1 K equals a change of 1 oC. To convert from degrees Celcius into kelvin add 273.15:- K C 273.15 Definition Absolute Zero is the lowest possible temperature, and something at this temperature has the lowest possible internal energy. This is zero kelvin, written 0 K, on the thermodynamic temperature scale. Definition The internal energy of an object is the sum of the random distribution of the kinetic and potential energies of its molecules. Boyle’s Law Temperature and moles of gas are constant Graph is hyperbolic (see below) and asymptotic to both axes Pressure and volume are inversely proportional to each other 1 p V Ideal Gas vs. Perfect Gas Strictly speaking an ideal gas is one that obeys Boyle’s law with complete precision. A perfect gas is a real gas under conditions that Boyle’s law is a valid enough description of its behaviour. Charles’ Law Pressure and moles of gas are constant Graph is linear (see right) Volume and temperature are directly proportional to each other V T Pressure Law (Gay-Lussac's Law ) Volume and moles of gas are constant Graph is linear (see right) Pressure and temperature are directly proportional to each other p T