4.1 Applications of Separation of Variables
advertisement
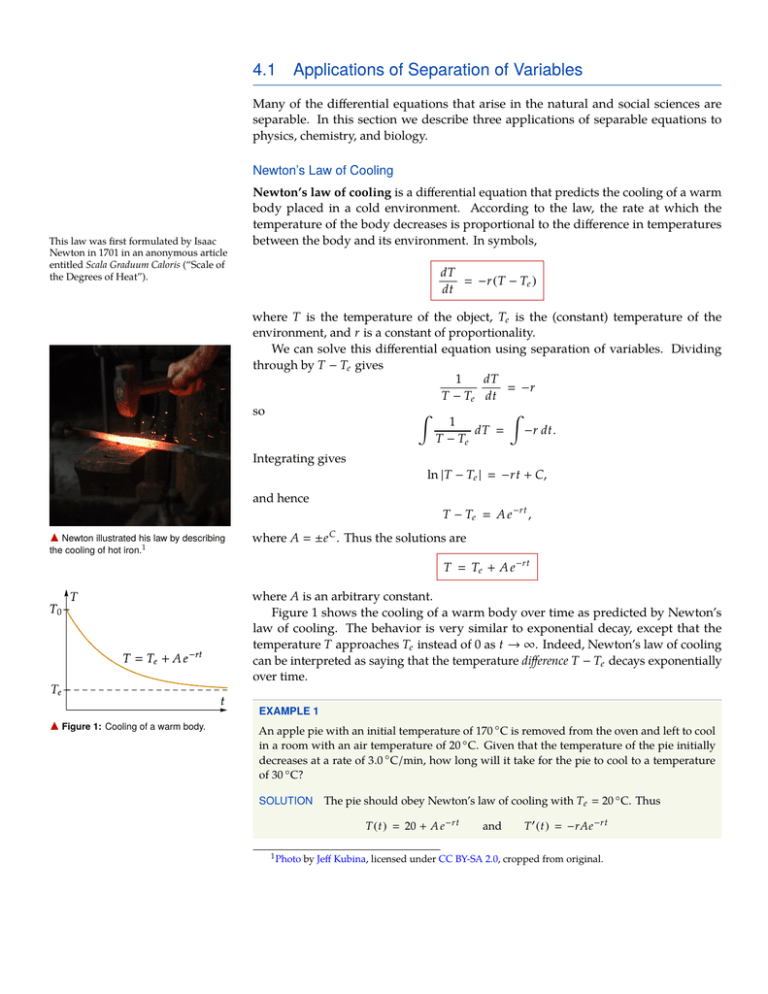
4.1 Applications of Separation of Variables Many of the differential equations that arise in the natural and social sciences are separable. In this section we describe three applications of separable equations to physics, chemistry, and biology. Newton’s Law of Cooling This law was first formulated by Isaac Newton in 1701 in an anonymous article entitled Scala Graduum Caloris (“Scale of the Degrees of Heat”). Newton’s law of cooling is a differential equation that predicts the cooling of a warm body placed in a cold environment. According to the law, the rate at which the temperature of the body decreases is proportional to the difference in temperatures between the body and its environment. In symbols, dT −r (T − Te ) dt where T is the temperature of the object, Te is the (constant) temperature of the environment, and r is a constant of proportionality. We can solve this differential equation using separation of variables. Dividing through by T − Te gives 1 dT −r T − Te dt so Z Z 1 dT −r dt. T − Te Integrating gives ln |T − Te | −rt + C, and hence T − Te A e −rt , a Newton illustrated his law by describing the cooling of hot iron.1 where A ±e C . Thus the solutions are T Te + A e −rt where A is an arbitrary constant. Figure 1 shows the cooling of a warm body over time as predicted by Newton’s law of cooling. The behavior is very similar to exponential decay, except that the temperature T approaches Te instead of 0 as t → ∞. Indeed, Newton’s law of cooling can be interpreted as saying that the temperature difference T − Te decays exponentially over time. a Figure 1: Cooling of a warm body. EXAMPLE 1 An apple pie with an initial temperature of 170 ◦ C is removed from the oven and left to cool in a room with an air temperature of 20 ◦ C. Given that the temperature of the pie initially decreases at a rate of 3.0 ◦ C/min, how long will it take for the pie to cool to a temperature of 30 ◦ C? SOLUTION The pie should obey Newton’s law of cooling with Te 20 ◦ C. Thus T ( t ) 20 + A e −rt 1 Photo and T 0 ( t ) −rAe −rt by Jeff Kubina, licensed under CC BY-SA 2.0, cropped from original. APPLICATIONS OF SEPARATION OF VARIABLES 2 for some constants A and r. We also have the initial conditions T (0) 170 ◦ C T 0 (0) −3.0 ◦ C/min. and Plugging these in gives the equations 170 20 + A and −3 −rA, so A 150 ◦ C and r 0.02. Thus T 20 + 150 e −0.02t . a Figure 2: The temperature function T ( t ) in Example 1. The diagonal dashed line shows the initial 3.0 ◦ C/min rate of temperature decrease. To find how long it will take for the temperature to reach 30 ◦ C, we plug in 30 for T and solve for t. The result is that t 135 min, as shown in Figure 2. Reaction Rates The study of the rates at which chemical reactions occur is a branch of chemistry known as chemical kinetics. For more complicated reactions, the chemical kinetics can involve a system of differential equations, with one equation for each reactant. In chemistry, the rate at which a chemical reaction occurs is determined by a differential equation called a rate equation. For a reaction with a single reactant, the concentration C of the reactant obeys a rate equation of the form dC −rC n , dt where r is a constant called the rate constant, and n is a constant called the order of the reaction. Typically the order of the reaction is the same as its molecularity, i.e. the number of molecules of the reactant that must come together to react. For example, the decomposition of sulfuryl chloride SO2 Cl2 −→ SO2 + Cl2 . is a first-order reaction (n 1), since it involves the decomposition of a single molecule of SO2 Cl2 . On the other hand, the decomposition of nitrogen dioxide 2 NO2 −→ 2 NO + O2 . a A sample of nitrogen dioxide gas. 2 is a second order reaction (n 2), since it only occurs when two molecules of NO2 come together. First and second order reactions behave somewhat differently. The rate equation for a first-order reaction is dC −rC, dt which is an instance of the exponential decay equation. Thus the concentration of the reactant decays exponentially. A second-order reaction, on the other hand, is governed by the equation dC −rC 2 , dt We can solve this equation using separation of variables. Dividing through by C 2 gives C −2 2 Photo dC −r, dt by W. Oelen, licensed under CC BY-SA 3.0, via Wikimedia Commons APPLICATIONS OF SEPARATION OF VARIABLES 3 so the solutions are given by Z Z C −2 dC −r dt. Integrating gives −C −1 −rt + A a Figure 3: Concentration of a for some constant A, so second-order reactant. C 1 rt + B where B −A. Figure 3 shows the decrease in the concentration of a second-order reactant according to this equation. This decrease behaves somewhat differently than exponential decay, with the concentration decreasing very quickly at first but having a very long tail. In particular, the half-life of the reactant is very small at first but increases over the course of the reaction, as shown in Figure 4. a Figure 4: Halving times for the reactant in a second-order reaction. Note the increasing half-life. EXAMPLE 2 The decomposition of nitrogen dioxide (NO2 ) is a second-order reaction. During a chemistry experiment, nitrogen dioxide with an initial concentration of 0.20 M is decomposed at a high temperature. If 90% of the NO2 decomposes during the first ten seconds, what is the rate constant for the reaction? SOLUTION Figure 5 shows the concentration [NO2 ] of nitrogen dioxide in this example. We know that 1 , rt + B and we are given that [NO2 ] 0.20 M at t 0, and [NO2 ] 0.020 M (10% of 0.20 M) at t 10 sec. This information yields two equations: [NO2 ] 0.20 M a Figure 5: The concentration of NO2 in Example 2. 1 B and 0.020 M 1 . r (10 sec) + B Then B 5.0/M, so the rate constant r is 4.5/ (M · sec) . Logistic Population Growth a A colony of Paenibacillus dendritiformis bacteria.3 Consider a colony of bacteria growing in an environment with limited resources. For example, there may be a scarcity of food, or space constraints on the size of the colony. In this case, it is not reasonable to expect the population of the colony to grow exponentially—indeed, the colony will unable to grow larger than some maximum population Pmax . In the study of population ecology, the maximum number of organisms of a certain type that a given environment can support is known as the carrying capacity of the population. Exponential population growth is a reasonable model in situations where the carrying capacity Pmax is very large, but for environments with a limited carrying capacity other models must be used to predict population growth. The most common model for population growth in an environment with limited carrying capacity is the logistic equation: 3 Photo from the laboratory of Dr. Eshel Ben Jacob, licensed under CC BY-SA 3.0, via Wikimedia Commons APPLICATIONS OF SEPARATION OF VARIABLES 4 P dP kP 1 − dt Pmax The idea of this equation is that the factor on the right approaches zero as P gets close to Pmax , causing the population growth to taper off. It is possible to solve the logistic equation using separation of variables, though the calculation is a bit difficult. (See Solving the Logistic Equation on the next page.) The solutions have the form Pmax 1 + A e −kt P a Figure 6: Logistic population growth. where A is an arbitrary constant. Figure 6 shows the graph of a typical solution to the logistic equation. Note that the population grows quickly at first, but the rate of increase slows as the population reaches the maximum. As t → ∞, the population asymptotically approaches the carrying capacity, i.e. lim P ( t ) Pmax . t→∞ Logistic functions are sometimes called sigmoid functions because of the S-like shape of their graphs, though this term is also used more broadly to refer to any function whose graph has an S-like shape. The solutions to the logistic equation are known as logistic functions, and can be used to model any situation where a variable is growing but the growth is bounded above. EXAMPLE 3 A population of bacteria is undergoing logistic growth with a maximum population of 100,000. Initially the population is 16,000. By t 1.0 hour, the population has grown to 64,000. What will the population be at t 2.0 hours? SOLUTION We know that 100,000 1 + A e −kt for some constants A and k. Plugging in the two data points gives P (t ) 16,000 100,000 1+A and 64,000 100,000 . 1 + A e −k Solving for A in the first equation gives A 5.25. Plugging this into the second equation and solving for k gives k 2.2336. Then a P (2) Figure 7: The bacteria population in Example 3. 100,000 94,315.8, 1 + (5.25) e − (2.2336)(2) so the population at t 2.0 hours will be approximately 94,000, as shown in Figure 7. EXERCISES 1. A bottle of water with an initial temperature of 25 ◦ C is placed in a refrigerator with an internal temperature of 5 ◦ C. Given that the temperature of the water is 21 ◦ C ten minutes after it is placed in the refrigerator, what will the temperature of the water be after one hour? 2. For the logistic population model, find a formula for the constant A that appears in the solution in terms of the maximum population Pmax and the initial population P0 . APPLICATIONS OF SEPARATION OF VARIABLES 5 A Closer Look Solving the Logistic Equation It is possible to solve the logistic equation using separation of variables. Starting with the equation dP P , kP 1 − dt Pmax we can separate the variables to get Pmax dP k. P ( Pmax − P ) dt Thus the solutions are given by Z Pmax dP P ( Pmax − P ) Z k dt. (1) The integral on the left requires the integration technique known as partial fractions decomposition. The idea of partial fractions decomposition is to write a complicated fraction as a sum of simpler fractions. In this case, observe that 1 1 Pmax + . P ( Pmax − P ) P Pmax − P This decomposition allows us to evaluate the integral: Z Pmax dP P ( Pmax − P ) Z 1 dP + P Z 1 dP Pmax − P ln |P| − ln |Pmax − P| + C P + C ln Pmax − P Plugging this into equation (1) and integrating the right side gives P kt + C. Pmax − P ln Then P A e kt Pmax − P and solving for P gives P where B 1/A. Pmax , 1 + B e −kt 3. In 1974, Stephen Hawking discovered that black holes emit a small amount of radiation, causing them to slowly evaporate over time. According to Hawking, the mass M of a black hole obeys the differential equation dM k − 2 dt M where k 1.26 × 1023 kg3 /year. (a) Use separation of variables to find the general solution to this equation (b) After a supernova, the remnant of a star collapses into a black hole with an initial mass of 6.00 × 1031 kg. How long will it take for this black hole to evaporate completely? APPLICATIONS OF SEPARATION OF VARIABLES 6 4. The decomposition of hydrogen iodide is a second-order reaction: 2 HI −→ H2 + I2 Initially the concentration of a sample of hydrogen iodide is 0.250 M, and the concentration is decreasing at a rate of 1.00 × 10−4 M/sec. (a) How long will it take for half of the hydrogen iodide to be consumed? (b) How long will it take for three quarters of the hydrogen iodide to be consumed? 5. The velocity of an object moving through a fluid can be modeled by the drag equation dv −kv 2 dt where k is a constant. (a) Find the general solution to this equation. (b) An object moving through the water has an initial velocity of 16 m/sec. After 2.0 seconds, the velocity has decreased to 12 m/sec. What will the velocity be after ten seconds? 6. A population of bacteria is undergoing logistic growth, with a maximum possible population of 100,000. Initially, the bacteria colony has 5,000 members, and the population is increasing at a rate of 400/minute. (a) How large will the population be 30 minutes later? (b) When will the population reach 80,000? 7. Water is being drained from a spout in the bottom of a cylindrical tank. According to Torricelli’s law, the volume V of water left in the tank obeys the differential equation √ dV −k V dt where k is a constant. (a) Use separation of variables to find the general solution to this equation. (b) Suppose the tank initially holds 30.0 L of water, which initially drains at a rate of 1.80 L/min. How long will it take for tank to drain completely? 8. The Gompertz equation has been used to model the growth of malignant tumors. The equation states that dP kP (ln Pmax − ln P ) dt where P is the population of cancer cells, and k and Pmax are constants. (a) Use separation of variables to find the general solution to this equation. (b) A tumor with 5000 cells is initially growing at a rate of 200 cells/day. Assuming the maximum size of the tumor is Pmax 100,000 cells, how large will the tumor be after 100 days?