Chapter #11 Notes
advertisement
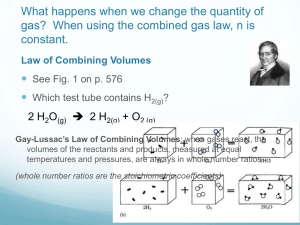
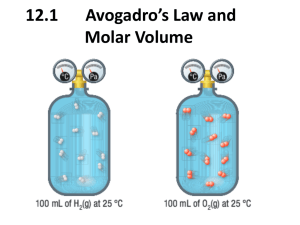
Chapter #11 Molecular Composition of Gases Chapter 11.1 • Gay-Lussac’s law of combining volumes of gases states that at constant temperature and pressure, the volumes of gaseous reactants and products can be expressed as ratios of small whole numbers. • Avogadro’s law states that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. • Standard Molar Volume of a gas is the volume occupied by one mole of a gas at STP. • 1 mole = 22.4 L Chapter 11.2 The ideal gas law PV=nRT Temperature must be in Kelvin!!! R is the gas constant 62.4 or 0.0821 you have to use the units of Pressure to pick which one… So… if pressure is in mmHg R=62.4 If pressure is in atm R= 0.0821 Molar mass or Density M= mRT PV D= MP RT M= DRT P M= Molar Mass m= mass in grams R= gas constant T= Kelvin Temperature P= Pressure V= Volume D= Density Chapter 11.3 • Stoichiometry of Gases • Volume to volume calculations • Liter to Liter same as Mole to Mole Mole to mole ratio from balanced equation • Given # of Liters x what you want what you have = Liters of what you want Chapter 11.4 • Graham’s law of Effusion states that the rates of effusion of gases at the same temperature and pressure are inversely proportional to the square roots of their molar masses. • A is the lighter gas, B is the heavier one. Work Cited • “Cartoon”. August 11, 2006. http://www.sapphireblue.com/catgoddess/farside .gif • “Gay-Lussac’s Law”. February 13, 2007. http://www.pinkmonkey.com/studyguides/subject s/chem/chap6/c0606501.asp • “Avogadro’s Law”. February 13, 2007. http://www.chem.ufl.edu/~itl/2045/matter/FG10_ 010.GIF • “Grahams Law”. February 15, 2007. http://www.humboldt.edu/~rap1/ChemSupp/Equ ations/EffusRatio.gif