Isotopes, e- config
advertisement

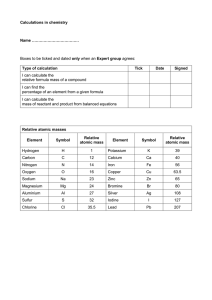
Isotopes, e- config How can hydrogen have an atomic mass that is 1.00794? How is atomic mass calculated? Hydrogen Isotopes Isotope- atoms of the same element with different numbers of neutrons Answer Protium makes up 99.98% of all hydrogen atoms Atomic Mass is average mass based on abundance Electron Configuration Electrons are distributed in energy shells around the nucleus. Each shell holds a certain amount of electrons Why? Energy Shell Electrons 2/8/8/2 for the first 20 elements Practice Example: Lithium, Atomic #3 Sulfur, #16 Carbon, # 6 Calcium, # 20 Now-Just the Outer Shell! Lewis Dot Structure: Carbon Lithium: Sulfur: Calcium: