Practice Problems 1405 Chapter 4.doc
advertisement
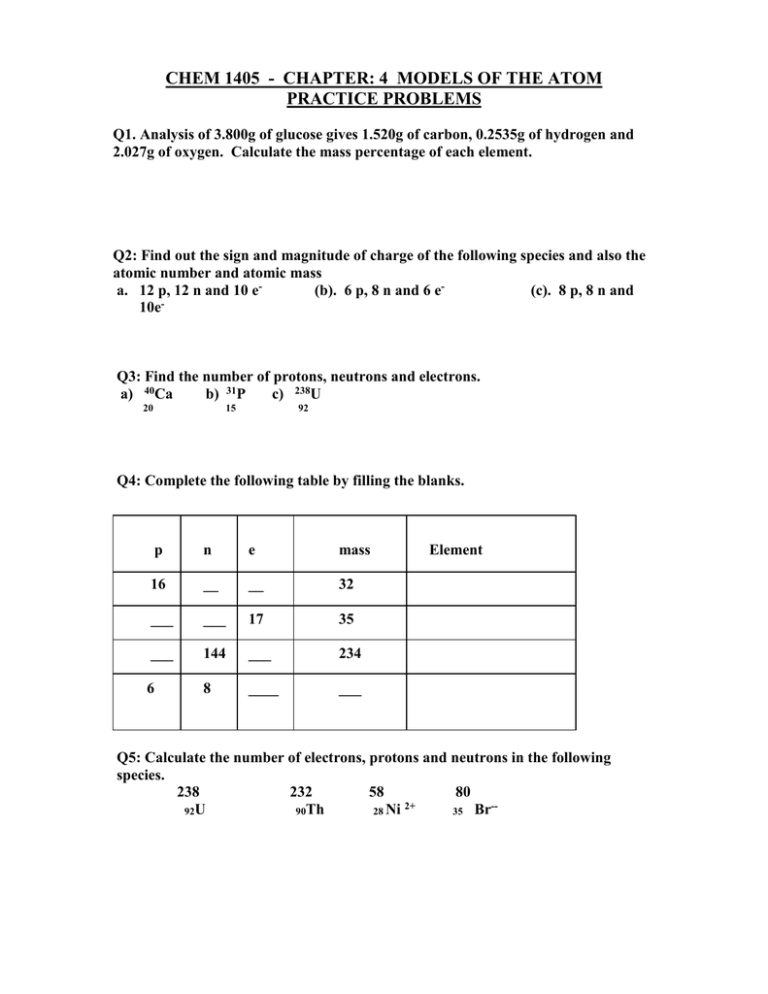
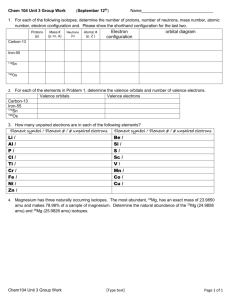
CHEM 1405 - CHAPTER: 4 MODELS OF THE ATOM PRACTICE PROBLEMS Q1. Analysis of 3.800g of glucose gives 1.520g of carbon, 0.2535g of hydrogen and 2.027g of oxygen. Calculate the mass percentage of each element. Q2: Find out the sign and magnitude of charge of the following species and also the atomic number and atomic mass a. 12 p, 12 n and 10 e(b). 6 p, 8 n and 6 e(c). 8 p, 8 n and 10e Q3: Find the number of protons, neutrons and electrons. a) 40Ca b) 31P c) 238U 20 15 92 Q4: Complete the following table by filling the blanks. p n e mass 16 __ __ 32 ___ ___ 17 35 ___ 144 ___ 234 6 8 ____ ___ Element Q5: Calculate the number of electrons, protons and neutrons in the following species. 238 232 58 80 2+ -92U 90Th 28 Ni 35 Br Q6: Calculate the average atomic mass of Ne, which is composed of three different isotopes of atomic masses 20.00 amu, 21.00 amu and 22.00 amu. The natural abundance of each isotope is 90.92%, 0.257% and 8.82% respectively. Q7: Mg consists of three isotopes of masses 24.0 amu, 25.0 amu and 26.0 amu. With abundances of 78.70%, 10.13% and 11.17% respectively. Calculate the average atomic mass of Mg. Q8. How many electrons are there in a p sub shell? Q9. What is the maximum number of electrons in a d sub shell? Q10. What happens to the energy of shells as it goes farther away from the nucleus? Q11. Find the number and type of sub shells in the 4th and 5th shell of an atom. Q12: How many and what kind of sub shells are allowed in the energy shell with principal quantum number 5? Also, calculate the maximum number of electrons. Q13. Find the electronic configuration and the core configuration of the following elements. Carbon, Sodium, Aluminum, Phosphorus, Nitrogen, Neon, Oxygen, Scandium, Iron, Chromium and Copper Q14: Name the elements described by the following electronic configuration. a) He 2s2,2p1 b) Ne 3s2,3p5 c) Q15: Write down the electronic configuration of K+ , Cl - , Ar Ca 2+ Q16: Find compact electronic configuration for the following elements. a) Br b) S c) C d) Fe 4s2 , N 3-