Grade 9 Chemistry Workbook: Bonding, Equations, Periodic Table
advertisement

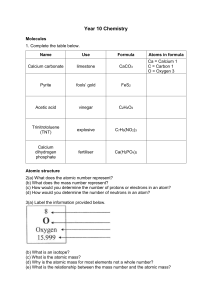
Beaconhouse School System GRADE 9 CHEMISTRY (5070) WORKBOOK Name: Wabbaj Muzaffar ……………………………………………………. Section: CB Chemical Bonding B2. Calcium is a metal and fluorine is a non-metal. a. Draw the atomic structure of an atom of calcium and of fluoride ion. [2] b. How does the atomic structure explain why calcium is a metal while fluorine is a non-metal. [2] Ca has 2 electrons in outermost shell meaning it belongs to G2 and ............................................................................................................................................. is a metal, while F has 7 electrons in its outermost shell meaning it ............................................................................................................................................. belongs to group 7 and is a halogen and forms electro negative ion. ............................................................................................................................................. c. Why is fluorine in group VII? [1] F has 7 electrons in outermost shell. ............................................................................................................................................. d. Why is calcium in period 4? [1] It has 4 shells for electrons. ............................................................................................................................................. e. Define the term relative atomic mass. [2] Average atomic mass of isotopes of an element is known ............................................................................................................................................. as relative atomic mass. ............................................................................................................................................. f. Deduce the electronic configuration of a calcium ion. [1] Ca*2+. ............................................................................................................................................. g. Explain, in terms of subatomic particles, why an atom of calcium is electrically neutral. It............................................................................................................................................. has the same number of electrons and protons as the opposite charges of the two cancel each other out ............................................................................................................................................. as the element Calcium becomes electrically neutral. ............................................................................................................................................. [3] Balancing Equations Balance these equations. 1. ___𝐹𝑒 + ___𝐻*𝑆𝑂/ → ___𝐹𝑒*(𝑆𝑂/)+ + ___𝐻*𝑂 2. ___𝐶𝑢𝑂 + ___𝐶 → ___𝐶𝑢 + ___𝐶𝑂* 3. ___𝐶/𝐻<= + ___𝑂* → ___𝐶𝑂* + ___𝐻*𝑂 4. ___(𝑁𝐻/)*𝐶𝑟*𝑂@ → ___𝐶𝑟*𝑂+ + ___𝐻*𝑂 + ___𝑁* 5. ___𝑁𝑎*𝐶𝑂+ + ___𝐻𝐶𝑙 → ___𝑁𝑎𝐶𝑙 + ___𝐶𝑂* + ___𝐻*𝑂 6. ___𝑁*𝐻/ + ___𝑂* → ___𝐻*𝑂 + ___𝑁* Periodic Table Section A 1. 2. 3. 4. 5. 6. 7. 8. Na is G1 metal while Mg is G2 metal, hence, D is correct option. 9. 1. 10. 2. 3. 12. Section B 1 electron Its is a good electrical conductor. R b h a s a l o w e r b o i l i n g a n d m e l t i n g p o i n t c o m p a r e d t o F e , a n d R b i s l e s s d e n s e a s w e l l a s s o f t e r t h a n F e Rb would react vigorously with water as bubbles of hydrogen gas will be given off, heat and light energy will be released as the reaction will take place. Yellow. Rb + 2H2O RbOH + H2. Lithium Sodium Potassium Rubidium Wear safety goggles and equipment for protection. RbPO*3- 4 Cesium