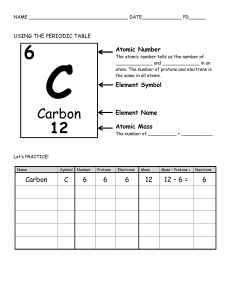
LECTURE ASSIGMENT #2 CHAPTER 2 AND 3 Professor Shirin Safaee Paula Cantú 1. The hydrogen atoms of a water molecule are bonded to the oxygen atom by a positive charge, in the case of water hydrogen, whereas neighboring water molecules are held together by negative charge, in the case of water by oxygen. 2. A solution of pH 5 contains 1000 times more H + than the same amount of a solution at pH 8. 3. What are the symbol, atomic number, atomic mass, and number of electrons in the last shell of the following atoms? Hydrogen: Symbol: H Atomic number: 1 Atomic mass: 1.008 Number of electrons in the last shell: 1 Oxygen: Symbol: O Atomic nunmber: 8 Atomic mass: 15.999 Number of electrons in the last shell: 6 Carbon 14: Symbol: 14C Atomic number: 6 Atomic mass: 14 Number of electrons in the last shell: 4 Nitrogen: Symbol: N Atomic number: 7 Atomic mass: 14.007 Number of electrons in the last shell: 5 Sodium: Symbol: Na Atomic number: 11 Atomic mass: 22.990 Number of electrons in the last shell: 8 Chlorine: Symbol: CL Atomic number: 17 Atomic mass: 35.43 Number of electrons in the last shell: 7 4. What is the different between ionic bond and polar covalent bond? Give an example for each one. The difference is that in the polar covalent bond the atoms have different electronegativity, so the atom with more electrons attracts the other strongly, resulting in those shared electrons “being more time” nearest it. This way, the ones that pulls harder have a minimum negative charge, meanwhile the one being shared has a positive charge. A clear example is water. Oxygen pulls harder because of its electronegativity is bigger than hydrogen, so they spend more time near oxygen. (Oxygen negative charged, Hydrogen positive charged). In contrast, ionic compounds are formed because of the transfer of one or more valence electrons from the positive charged atom to an atom with negative charged. (opposite charged). Example: sodium fluoride (NaF) In this case, sodium loses its only electron. The main difference is that in covalent they SHARE; in ions they TRANSFER valence electrons. 5. What are the building units (monomers), functions and examples (3 examples for each one) of the four Macromolecules? Carbohydrates: monosaccharides. Give instant energy, regulation of blood glucose and energy storafe. Bread, pasta and fruits. Proteins: amino acids. Growth and Maintenance, structure and energy. Chicken, whole milk, steak. Lipids: glycerol, fatty acids store energy, absorb vitamins and production of hormones. Olive oil, nuts and butter. Nucleotides; DNA storage of chemical energy, It stores instructions for the production of protein and gene expression fish, seafood, legumes, and mushrooms 6. In medicine we use artificial hormones to treat disease. Those medicine may have side effect and there is potential for abuse. Explain the uses, abuse, and side effects of anabolic steroids. They can be used for medical reasons but in 2023, is used primarily for aesthetic and bodybuilders. They have said effects because of the imbalance of the hormone. it can lead to reduce sperm, depression and hair loss. People aren’t aware of the side effects and can lead to unhealthy body for seeking a good physical appearance. They should always be in hand with a doctor and nutritionist and not exceed the recommended doses.