DO NOW MONDAY
advertisement

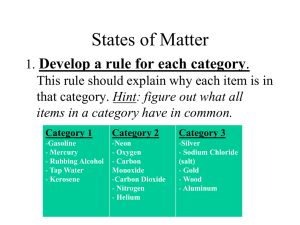
DO NOW MONDAY • Find your new assigned seat. • Write one thing (or more) that you enjoyed most about your Christmas break. • Today’s PLAN – To be prepared for second semester in 8th Grade Science – To illustrate Mastery on first semester 8th Grade Learning Targets • Todays DO – We will review CLASS EXPECTATIONS – We will look at and retake semester test (if necessary) and/or do enrichment over first semester learning targets – We will prepare our binders for second semester CLASS EXPECTATIONS 1) Be on time. 2) Be prepared for class before class begins. 3) Sit down and begin work on your DO NOW as soon as you enter the classroom. 4) Speak ONLY when given permission. 5) Get up out of your seat ONLY when given permission. Enrichment Directions • Work with a partner. • Go to my teacher website from the BCMS homepage. • Click on the Gizmos Website link under Files/Links. • Click on Login/Enroll at the top of the page. • Username is dyerclass1 • Password is bcms2015 • Click on Find Gizmos at the top of the page. • Type in Mouse Genetics • Click on Mouse Genetics (One Trait) • Click on Launch Gizmo. • Follow the directions and complete the questions in the packet. Tuesday, January 5th Do Now: • Copy the Learning Targets in your binder: “I can… Target 1: Matter 1.1 Contrast matter energy 1.2 Classify matter by phase and composition 1.3 Describe energy transfers associated with phase changes Concept Connection Map: • Word List: principal math teacher homework school students Barren Middle Pre-Assessment: Concept Mapping Activity • Create a concept map using the following words: • • • • • • • Matter Chemistry Solid Liquid Gas Melting Freezing • • • • • Temperature Energy Mass Volume Density Taking Notes: please copy… • Matter is anything that has mass and occupies space Describing Matter: • How would you describe the table? • How could you describe your chair? Properties (Characteristics) of Matter – •how it is described •Hot, cold, hard, soft, rough, smooth, shiny, dull, solid, liquid, gas, etc. •Boiling Point, Melting Point, Freezing point Phases of Matter • Phases (states) of matter: – Tells us about the arrangement and motion of the particles that make up the matter • • • • Solid Liquid Gas Plasma Matter: Video Clip • While viewing consider the states of matter. http://www.youtube.com/watch?v=guoU_cuR8EE&feature=related http://www.youtube.com/watch?v=xYFAj50c7xM Phases of Matter Notes • You will take notes over the four states of matter on the graphic organizer (chart) given to you. Solid • Particles (atoms or molecules) are packed closely together and stay in a fixed position • Movement consists of vibrating particles staying in place • Two types of Solids – Crystalline Solid – particles in a distinct pattern, melt at a specific distinct temperature. Examples include sugar, salt, ice, quartz, etc. – Amorphous Solid – particles arranged in an irregular pattern, therefore no real distinct melting point. As heat is applied to an amorphous solid, the substance changes from hard to softer and softer until a liquid. Examples include rubber, plastic, glass, and chocolate! Liquid • No shape of its own, takes on the shape of the container, but it has a definite volume • Particles in a liquid – atoms are loosely bound and are free to flow and move, sliding easily over , under, and across each other but remaining in contact w/ one another. • Viscosity – resistance of a liquid to flow: – high viscosity: “thick”; slow flowing (molasses in January) – low viscosity: “thin”; fast flowing (pouring water) Gas • No shape of its own, takes the shape of the container it is in. • No definite volume, easily compressed Measuring Gases – • Three important measurements taken for gases. All three are closely related!! Volume Temperature & Pressure • Volume – Gases dissipate to evenly fill the container they occupy • Temperature • Pressure – 1. The measurement of the average thermal energy of the particles in the gas. – 2. The average speed of a gas molecule at room temperature is fast!! 500 meters per second! – – gas particles are in constant motion and exert pressure upon the container they occupy. Because the gas particles are in motion, they collide and bounce off each other and the sides of the container. This contact w/ the sides of the container causes and outward push. Pressure = Force Area Plasma • State of matter that has had the electrons stripped away • Particle move extremely fast – Fire is in the Plasma state – Glow around re-entry vehicles from space – The Sun Phases of Matter Exit Slip: • Must all things fall into the category of solid, liquid, gas, plasma? Draw, define, & example: • • • • • • Chemistry Evaporation Vaporization Condensation Sublimation Matter Changes in Matter • Physical Change -A change that alters the form of a substance but not the chemical makeup of the substance, a change of state – Words like: crush, smash, tear, evaporate, slice, breakdown, dissolve, absorb, swell, burst • Chemical Change - One or more substances combine or decompose to form a chemically different substance – Words like: react, burns, forms, decomposed, rusting, sours, rotting, digesting, cooked, molecular change Types of Matter • Pure Substances – Those substances made up of one kind of matter. It has definite characteristic properties – Elements: Contain only one type of atom, H, He, Na, Mg, C, N, O, – Compounds: A pure substance formed by the chemical combination of two or more elements - CO2, H2O, C6H12O6, NaCl • Mixed Substances (Mixtures)– two or more substances that are mixed together but not chemically combined. – Homogeneous Mixture: a very well mixed mixture -solution of sugar water – Heterogeneous Mixture: not evenly mixed - handful of dirt, Rocky Road Ice Cream, Measuring Matter • SI – International System of Units = the metric system – Length – the one dimensional measurement of distance – SI unit is Meter, Kilometer – Mass – the amount of matter in a substance – SI unit: gram or kilogram – Weight – the force of gravity acting on an object – SI unit: Newton – Volume – how much space an object occupies – SI unit: liter, milliliter, cm3 • Solid Volume = Length x Width x Height = cm3, meter3 • Liquid Volume = liter, milliliter • 1ml = 1cm3 – Density – the amount of mass an object has in a given volume – SI unit: g/ml, g/cm3 • Density= Mass / Volume – Temperature – the average kinetic energy of an object. • 0C = Centigrade or degrees Celsius, 0K = degrees Kelvin • 0 0C = 273 0K – Time: unit of measure: second, minute Measuring Matter Particles of Matter • Atoms – The smallest particle of an Element that retains the chemical properties of that element • Democritus – 400 BC, a Greek philosopher that coined the term “atomos” which means “uncuttable, indivisible” John Dalton -1802 - The Atomic Theory • Ding-a-Ling!! Ding-a-Ling!! • Atoms can not be broken into smaller pteces – atoms are like a solid marble (Not entirely accurate) • In an element all atoms are exactly alike (Not entirely accurate) • Atoms of two or more elements can combine to form compounds ( this is true) • Atoms of each element have a unique mass (Not entirely accurate) • Compounds are always composed of whole number proportions of elements ie CO2 – Carbon dioxide, H2O – Water, C6H12O6 – Glucose, NaCl – Table Salt (this one is true also) The basic particle of an Element is the Atom – H, He, Fe, etc The basic particle of a Compound is the Molecule – a group of atoms that are chemically bonded and act as a single unit until the bonds are broken: CO2, H2O, C6H12O6, NaCl