Chemical Families Name__________________________ Period________Date______________
advertisement

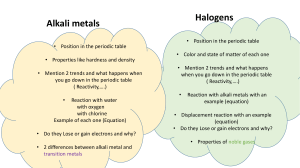
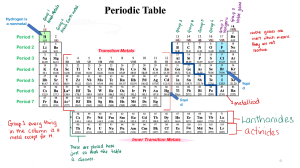
Chemical Families Name__________________________ Period________Date______________ Word List: Some words may not be used. alkali metals 500 move dull phosphorous I Cs halogens noble gases Mg shiny 100 gaseous electrons S less Li family neutrons reactive gases ductile protons Hg Rb decreases K malleable Br period 200 conduct families more Na increases inert gases 1. There are more than ____________ elements. 2. Metals are _________________ in appearance. 3. Metals are also ____________________, meaning that they bend without breaking. 4. Metals ____________________ heat and electricity. 5. The alkali metals include _______, _______, _______, _______, and _______. 6. One example of a nonmetal would be _________. 7. At room temperature this element is a liquid: ___________. 8. The atomic number is the same as the number of _________________ in a neutral atom. 9. Elements that exhibit similar behavior are classified in the same ___________________. 10. Ar, He, Kr, Ne and Xe are known collectively as the ________________________ or the _______________________. 11. F, Cl, Br, and __________ make up the ____________________ group. 12. In the Periodic Table of the Elements, _____________________ are arranged vertically. 13. The alkali metals have one ______________ electron that the Noble Gases. 14. The number of electrons __________________ as you go from left to right on the Periodic Table.