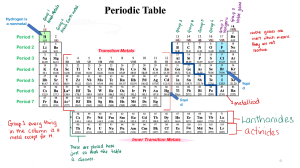
Word List: Not all words are used. electrons S I Mg ductile K 500 less Cs Shiny protons malleable move Li Halogens 100 Hg Br dull Family neutrons gaseous Rb Period conduct Na families more Increases inert gasses reactive gasses Alkali metals Decrease 200 Phosphorous Noble gasses 1. There are more than ____________ elements. 2. Metals are _________________ in appearance. 3. Metals are also ____________________, meaning that they bend without breaking. 4. Metals ____________________ heat and electricity. 5. The alkali metals include _______, _______, _______, _______, and _______. 6. One example of a nonmetal would be _________. 7. At room temperature this element is a liquid: ___________. 8. The atomic number is the same as the number of _________________ in a neutral atom. 9. Elements that exhibit similar behavior are classified in the same ___________________. 10. Ar, He, Kr, Ne and Xe are known collectively as the ________________________ or the _______________________. 11. F, Cl, Br, and __________ make up the ____________________ group. 12. In the Periodic Table of the Elements, _____________________ are arranged vertically. 13. The alkali metals have one ______________ electron that the Noble Gases. 14. The number of electrons __________________ as you go from left to right on the Periodic Table