STATES OF MATTER NOTES 12/06/05-12/11/05
advertisement

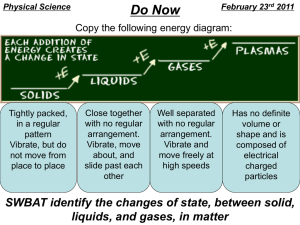
STATES OF MATTER NOTES 12/06/05-12/11/05 A) THE SOLID STATE: a. Solids do not assume the shape and volume of the container. b. Solids are the lowest energy state for a specific substance. c. Attractions are strongest in the solid and thus the particles have little freedom of motion and randomness (entropy). d. The particles are closest in the solid state except for water which has Hydrogen bonding. e. Non polar covalent small substances with relatively weak Van der Waals have the lowest freezing points (and boiling points) relative to other substances. f. Polar covalent substances with dipole attractions have low freezing and low boiling points. g. Ionic substances with electrostatic attractions have high freezing and boiling points. h. The freezing and boiling boiling points of metallic substances vary with the heavy metals having the highest boiling points (due to Van Der Waals getting stronger with mass).. i. This part of the graph is a slope due to a kinetic energy change (temperature is average Kinetic energy measurement) B) THE MELTING/FREEZING PHASE CHANGE a. The equilibrium between the solid and liquid states at the freezing (melting) temperature point. b. This part of the graph is a plateau because this is a potential energy change. c. If the temp is increasing (heating curve) the equilibrium favors melting. d. If the temp. Is decreasing (cooling curve) the equilibrium favors freezing. e. The LOWER ENERGY PLATEAU is the freezing/melting point. f. The attractions are weakening as heat is added (endothermic) and stored in the substance, more freedom of motion and entropy results. g. The attractions get stronger as heat is removed (exothermic), less freedom of motion and entropy. C) LIQUID PHASE a. The attractions are weaker, motion is greater and entropy is greater. b. Liquids assume the shape of the container, but NOT the volume. c. Liquids have fluidity, particles can move and flow. d. Liquids produce VAPOR PRESSURE as they evaporate into the gas phase. e. The attractions between liquid particles cause cohesion, while the. attraction of the liquid to the container is called adhesion D)BOILING/CONDENSATION PHASE EQUILIBRIA. f. The equilibrium of the liquid and gas phases at the boiling point temperature. g. Attractions are assumed to be totally broken. h. The newly formed gas is called VAPOR and is in contact with the liquid surface. i. This is the higher temperature plateau, potential energy changes and kinetic is constant. j. Strong attractions correspond to high boiling points, IONIC > POLAR COVALENT > NONPOLAR k. In order to boil, a liquid must have enough energy to 1) Break attractions 2) Overcome atmospheric pressure. l. A liquid can never go above its boiling point, unless pressure is increased. j. Remember both condensation and evaporation occur at the same time, if you are heating evaporation is faster and the liquid boils. If you are cooling, the liquid condenses. E) GAS PHASE a. Particles have no attractions. b. Particles move in random straight line motion. c. Particles collide with each other and exchange energy. d. Particles have negligible volume, a gas system is considered to be empty space. e. The volume of a gas will change with pressure and temperature. f. Gasses always assume the volume and shape of the container. g. When working with gasses, we use the absolute (Kelvin) scale of temperature. h. CHARLES LAW; the volume of any gas will increase in direct proportion to the temperature increase. The volume of any gas will decrease in direct proportion to a temperature decrease. This is at constant pressure in an expandable container. i. BOYLES LAW: the volume of a gas will decrease as pressure applied to the gas increases (inverse proportion). The volume of a gas will increase as pressure applied decreases. This is at constant temperature in an expandable container. j. LAW OF GAY-LUSSAC: as the temperature of a closed non expandable gas system increases the pressure increases (direct proportion). k. GRAHAMS LAW: gasses with the largest molar masses effuse slowest, gasses with low molar masses effuse fastest.