2.3 Review & Reinforcement Name________________________________ Period______Date______________________
advertisement

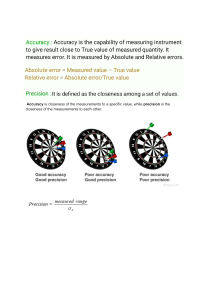
2.3 Review & Reinforcement Name________________________________ Uncertainty in Measurement Period______Date______________________ 1. Define the terms precision and accuracy. Explain the difference between the terms using an example. ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ 2. Why are measurements always uncertain? ________________________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ 3. Name at least four kinds of measurements that are frequently made in the laboratory. ___________________________________________________________________________________ 4. Why is estimation a necessary part of making measurements? ________________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ 5. What is the “standard” (accepted value) in a game of darts? __________________________________ 6. Explain the phrase, “an instrument is only as good as its operator.” ____________________________ ___________________________________________________________________________________ ___________________________________________________________________________________ 7. What factors determine the precision of an instrument? ______________________________________ ___________________________________________________________________________________ 8. What variable determines the accuracy of an instrument? ____________________________________ 9. Read the level of the liquid in each of these graduated cylinders to the nearest 0.01 mL. Remember to read the volumes at the meniscus. 10. An electronic balance shows the mass of a sample of sodium chloride to be 29.732 g. What is the uncertainty of the measurement? _________________ In what range can the true value exist? __________________________________________________________________________________