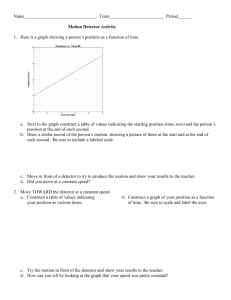
Document 13938094
advertisement
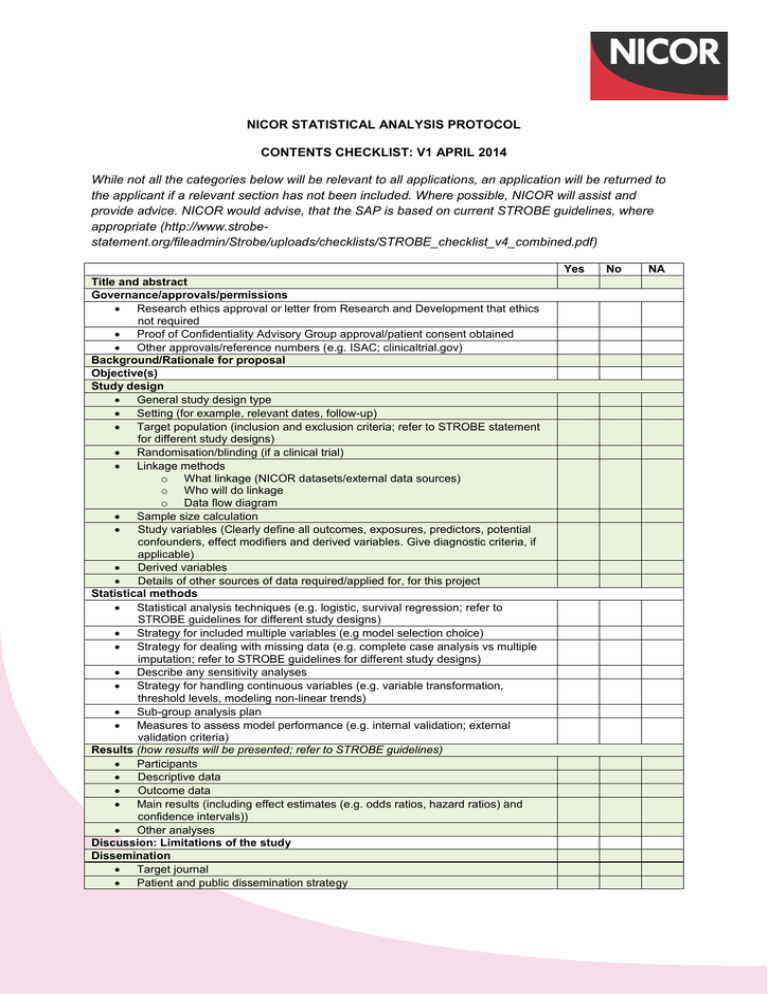
NICOR STATISTICAL ANALYSIS PROTOCOL CONTENTS CHECKLIST: V1 APRIL 2014 While not all the categories below will be relevant to all applications, an application will be returned to the applicant if a relevant section has not been included. Where possible, NICOR will assist and provide advice. NICOR would advise, that the SAP is based on current STROBE guidelines, where appropriate (http://www.strobestatement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf) Yes Title and abstract Governance/approvals/permissions Research ethics approval or letter from Research and Development that ethics not required Proof of Confidentiality Advisory Group approval/patient consent obtained Other approvals/reference numbers (e.g. ISAC; clinicaltrial.gov) Background/Rationale for proposal Objective(s) Study design General study design type Setting (for example, relevant dates, follow-up) Target population (inclusion and exclusion criteria; refer to STROBE statement for different study designs) Randomisation/blinding (if a clinical trial) Linkage methods o What linkage (NICOR datasets/external data sources) o Who will do linkage o Data flow diagram Sample size calculation Study variables (Clearly define all outcomes, exposures, predictors, potential confounders, effect modifiers and derived variables. Give diagnostic criteria, if applicable) Derived variables Details of other sources of data required/applied for, for this project Statistical methods Statistical analysis techniques (e.g. logistic, survival regression; refer to STROBE guidelines for different study designs) Strategy for included multiple variables (e.g model selection choice) Strategy for dealing with missing data (e.g. complete case analysis vs multiple imputation; refer to STROBE guidelines for different study designs) Describe any sensitivity analyses Strategy for handling continuous variables (e.g. variable transformation, threshold levels, modeling non-linear trends) Sub-group analysis plan Measures to assess model performance (e.g. internal validation; external validation criteria) Results (how results will be presented; refer to STROBE guidelines) Participants Descriptive data Outcome data Main results (including effect estimates (e.g. odds ratios, hazard ratios) and confidence intervals)) Other analyses Discussion: Limitations of the study Dissemination Target journal Patient and public dissemination strategy No NA