NICOR DATA ACCESS PROCESS
advertisement

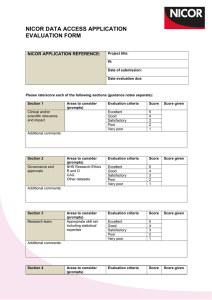
NICOR DATA ACCESS PROCESS NICOR DATA ACCESS EXPRESSION OF INTEREST/INFORMAL DISCUSSION Contact the appropriate project manager by email or telephone NICOR DATA ACCESS APPLICATION SUBMITTED (only latest version of the application is accepted) *Complete application: Forwarded for review. Incomplete application: Returned to the applicant (timeline 5 working days) DATA ACCESS REQUEST FOR DATA FROM ONE NICOR DATABASE REVIEWED BY THE AUDIT RESEARCH GROUP (meets monthly, dates available on the NICOR website) NOT APPROVED DATA ACCESS REQUESTS FOR LINKED NICOR DATA REVIEWED BY THE RESEARCH EXECUTIVE (meets every two months, dates available on the NICOR website) **APPROVED WITH MODIFICATIONS APPROVED APPEAL The PI can appeal within 4 weeks of notification NO PSEUDOANONYMISED OR IDENTIFIABLE DATA REQUESTED PSEUDOANONYMISED OR IDENTIFIABLE DATA HQIP approval required after NICOR approval has been given. NICOR will submit application to HQIP (timeline 1 month) APPROVED Notes *For an application to be considered, NICOR require: A completed application form; Evidence of Research Ethics Committee (REC) approval or R and D evidence that REC approval is not required; Evidence of Confidentiality Advisory Group (CAG) approval or patient consent, statistical analysis plan. **Major revisions will be reviewed by the original reviewing committee. Minor revisions may be approved by Chairman’s action. • • • • • NOT APPROVED REQUIREMENTS TO RECEIVE EXTRACT PI to sign NICOR data access agreement form Registration of study on clinicaltrials.gov (timeline dependent on PI) Preparation of data extract by NICOR (timeline 4 weeks from receiving above) POST NICOR APPROVAL Annual progress report Final study report