NICOR DATA ACCESS APPLICATION FORM NICOR REFERENCE
advertisement

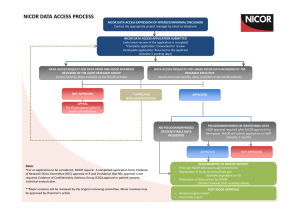
NICOR DATA ACCESS APPLICATION FORM NICOR REFERENCE (office use only) Please refer to NICOR data sharing policy (including charges for data extracts) before completing this application (www.ucl.ac.uk/nicor/dataforresearch). All sections must be completed. Incomplete forms will be returned which will cause a delay to the application being reviewed. All applications require submission of a data flow diagram and a statistical analysis plan. For applications requiring HQIP approval (please refer to NICOR’s data application process flow chart), please provide a copy of your organisation’s: o o o o Deletion policy Data and IT usage policy Data protection and security policy Confidentiality policy All research applications must register with clinicaltrials.gov http://clinicaltrials.gov/ prior to a data extract being provided by NICOR SECTION I: NICOR APPLICATION Project title: PI (Title, forename, surname): Date of submission: Has this application been previously submitted and rejected? If yes, please give details: SECTION II: NICOR DATASET EXTRACTS REQUESTED (Please indicate all that apply) SECTION III: LINKAGE OF NICOR ☐ ☐ ☐ ☐ ☐ ☐ ☐ National audit of Myocardial Ischaemia (MINAP) National Audit of Heart Failure National Audit of PCI National Audit of Adult Cardiac Surgery National Audit of Cardiac Rhythm Management National Audit of Congenital Heart Disease Transcatheter Aortic Valve Implantation registry Does your project involve linking NICOR dataset(s) with another database? Yes ☐ No ☐ (if no, go to next section) Name external dataset(s) NICOR data to be linked with: 1 DATASET EXTRACT TO ANOTHER DATASET a) b) c) Name of Trusted Third Party (TTP) who will conduct linkage: a) Organisation: b) Contact details: SECTION IV: GOVERNANCE Have you read, and will you be able to accept the terms and conditions for the NICOR (and HQIP, where applicable) data sharing agreement(s): Yes ☐ No ☐ Please provide your organisations UK Information Commissioner’s Office (ICO) registration number: Type of work applying for data for (must comply with the Health Research Authority definitions http://www.hra.nhs.uk/researchcommunity/before-you-apply/determine-whether-your-study-isresearch/): Research: ☐ Audit: ☐ Service evaluation: ☐ Ethics (for research applications only, if linkage with external NICOR dataset): Ethics reference (please attach copy of approval letter): R and D (for all types of application): R and D reference (please attach copy of letter): Confidentiality advisory group (CAG) (for applications requiring identifiable patient information without patient consent. More information can be found at http://www.hra.nhs.uk/resources/confidentiality-advisorygroup/): CAG reference (please attach copy of approval letter): Others (Please provide details of any other permissions gained and relevant reference): Clinicaltrials.gov registration (research applications only) Clinicaltrials.gov reference: SECTION V: FUNDING Do you have funding to conduct this work? Yes ☐ please state how you plan to fund this work) No ☐ (if no, 2 Name of funding body: Dates/duration of award: Title of application: Synopsis of application (max 100 words): SECTION VI: PRINCIPAL INVESTIGATOR Title, forename, surname: Employing organisation: Position in organisation: Address of organisation: Academic affiliation of PI: Telephone: Email: Please attach PI CV to your application Research team member 1 Title, forename, surname: Research team member 2 Title, forename, surname: Employing organisation: Employing organisation: Position in organisation: Position in organisation: Telephone: Telephone: Email: Email: Research team member 3 Title, forename, surname: Research team member 4 Title, forename, surname: Research team member 5 Title, forename, surname: Employing organisation: Employing organisation: Employing organisation: Position in organisation: Position in organisation: Position in organisation: Telephone: Telephone: Telephone: Email: Email: Email: SECTION VII: RESEARCH TEAM/ CO-INVESTIGATORS Please list the main publications of each research team member SECTION VIII: involved in the project: PUBLICATIONS OF THE RESEARCH TEAM (INCLUDING PI) SECTION IX: Summary (a brief summary of up to 200 words describing the aims of the work) 3 RESEARCH PROJECT Context (Where research is part of a larger programme, please give details): Project description (Full description of the purpose/s for which the data are requested (maximum 4 A4 sides excluding references). Please use the structure in the NICOR Statistical Analysis Protocol. This applies for all data sharing applications, whether submitted a request for data for research, audit or service evaluation work. The main categories are: Title Abstract Background/rationale for the proposal Objectives Study design (including data flow diagram for linkage studies) Statistical methods (including statistical analysis plan) Results Limitations of the work Dissemination (including planned scientific outputs and a patient and public dissemination strategy) Proposed project completion date: SECTION X: DATA REQUESTED Please refer to the audit datasets, available on the NICOR website Dataset items required (Please refer to the relevant dataset template (appendix 1-7)) Dataset time period: Geographical location (e.g. UK, England, Wales, etc.): Cleaned dataset ☐ raw data ☐ Annual updates required: Yes ☐ SECTION XI: DATA MANAGEMENT No ☐ Please state how the data will be stored and accessed: Please outline the period of retention: Please describe the IT infrastructure and network used to access and retrieve the data: Confirm if any portable/mobile media will be used (for example, laptop/ CD/disk to retain/access the data): 4 SECTION XII: PATIENT AND PUBLIC ENGAGEMENT Please provide details of the involvement of patients and the public in the design of this work. Please provide details on the relevance of the results to patients and the public Please provide a lay summary of your work (max 250 words) SECTION XIII: CONFLICTS OF INTEREST Please provide details of any conflicts of interest (for example, financial, consultancy and professional activities, clinical trials, equity holdings, Executive and non-executive directorships) of the PI or research team. SECTION XIV: SIGNATURES PI (organisation requesting the data): The Parties have signed to acknowledge that this Agreement shall apply to any Data shared by HQIP further to any Data Sharing Request Form completed by the Applicant. Name: NB: This agreement is not valid until all there organisation have agreed and signed: Signature: Position: Address: Email: Date of signature: NICOR Audit clinical lead (approval that the clinical lead is happy with the proposed use of data): Name: Position: Signature: Date of signature: NICOR approving committee Chair: Name: Position: 5 Signature: Date of signature: HQIP Name: Position: Email: Signature: Date of signature: 6