National Heart Failure Audit Steering Group meeting minutes 3 July 2014, 13.30-15.30
advertisement

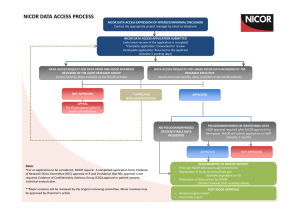
National Heart Failure Audit Steering Group meeting minutes 3 July 2014, 13.30-15.30 rd Boardroom, 3 floor 170 Tottenham Court Road, London W1T 7HA 1. Apologies and introductions 1.1. Present: Jackie Austin, Gemma Baldock-Apps, Janine Beezer, John Cleland, Dawn Lambert, Suzanna Hardman, Theresa McDonagh (chair), Richard Mindham, Polly Mitchell, Jim Moore, Julie Sanders, Aminat Shote, Kathy Simmonds. 1.2 In attendance: Morag Cunningham (TC) 1.3 Apologies: Gethin Ellis 2. Minutes of the last meeting and matters arising AP1: PM confirmed that Marion Standing had added life status information to the export, but due to delays at the HSCIC, no mortality data has been released to NICOR. AP3: TM noted that the BSH audit presentation slides were in a very large file, so there had been some complaints from a few people about the fact that they are available as pdf rather than PowerPoint. She has sent the file to a couple of people via the drop box. It was discussed whether to include a line on the BSH website explaining that you have to use the ‘print’ function to download the pdf file, or otherwise cut graphs out of the NICOR report. It was agreed that prospectively, a slide pack would be produced alongside the annual report for 2013/14, and uploaded to the NICOR website along with the report pdf. It was also agreed that the report summary should be downloadable as a separate pdf file from the main report. Action1: 2013/14 report to include slide pack with main analyses, and separate summary report. AP4: PM confirmed that there weren’t any hospitals currently trying to use CVIS to import data, due to the delays with Philips upgrade. Both hospitals that were previously using it have switched to direct data entry. After the next Philips upgrade later in the year, the number of hospitals using the CVIS HF module is likely to increase. AP6: PM, TM and JM had a TC to discuss proposed primary care linkage with Gloucestershire Care Services data. PM and JM have put together a proposal, which will be discussed at the next NICOR executive. There were some concerns about information governance, in particular whether NICOR can hold primary care PID data, which need to be addressed. Action 2: JM and PM to investigate whether the Gloucestershire secondary care HF team is ready to start retrospective data collection. 3. Annual report 2013/14 and analysis plan PM has circulated the analysis plan, including additional analyses for 2013/14, and an outline version of the 2013/14 report. PM noted that an analysis looking at length of stay against mortality had been discussed, but wasn’t currently included in the analysis plan. TM noted that this was included in the 2012/13 risk model, and did not show a U shaped relationship, as anticipated. It was suggested that an analysis looking at length of stay against 30-day readmission could be included instead. JC noted that early discharge could in some cases be due to high quality post-discharge management in the community, which might result in unexpected outcomes. It was suggested that this should be a combined 30-day readmission/mortality measure, but noted that 30-day mortality is measured from date of admission (i.e. including in-hospital mortality), whereas 30-day readmission was measured from date of discharge (i.e. only covering survivors to discharge). This tracks the CCG Outcome Indicator Set (OIS) measures. However it was decided that for this analysis the endpoint should be combined 30-day readmission/death from point of discharge, although this wouldn’t apply to any other 30-day mortality analyses. It was agreed that AS would look at LOS against 30-day readmission/death (from discharge), although this might not be included in the final version of the report. Action 3: PM to add LOS against 30-day all-cause readmission/mortality (from discharge) to 2013/14 analysis plan. PM went through the report outline briefly - main changes from 2012/13 included readmission analysis and fewer Kaplan-Meier graphs. There were no additional comments. It was noted that a draft needed to be submitted to HQIP by mid-September, with publication in midNovember. PM reminded the group that there would be a two-week validation of hospital level data prior to publication, which will be sent out to all HF contacts as soon as mortality data has been obtained. As long as delays to ONS/HES linkage are not too extensive, this timeframe was deemed to be achievable. Action 4: Hospital level data to be sent to all HF contacts once ONS linkage has occurred. 4. Best practice tariff update SH noted that there was a workshop on 4 July which she, TM and PM would be attending. There were several questions which needed addressing: • What percentage of patients would be expected to see a specialist; • Whether it would be possible to verify whether a patient had actually seen a specialist or not (i.e. to validate the data); • Whether analysis be required at hospital or CCG level; • Whether hospitals would have access to data before commissioners, or preferably whether commissioners could access data through hospitals; • Whether the BPT would actually provide a financial incentive for good practice, or just a financial disincentive for failing to meet standards. There was a general concern that this initiative could take funds out of HF care overall. PM said that the briefing paper for the workshop on 4 July included a couple of suggestions, some where there was a decrease if the tariff for failing to meet the target, and others where there was an increase in the current tariff for meeting best practice standards. A number of other concerns were raised: • Whether it’s feasible for hospitals to submit data with quarterly deadline - TM noted that it’s difficult enough to submit to an annual deadline for many Trusts. SH explained that because HF is a retrospective audit, database users have to access the notes for each patient following clinical coding, which can cause delays. • JS noted that Mike Richards (chief inspector of hospitals) is keen to discuss the ways of identifying and preventing ‘gaming’ in data entry with NICOR, and asked what kind of reports could be provided in HF. SH said that in 2013 the audit had noticed a rise in prescription of beta blockers, and in order to check whether this was a real increase, had looked at whether there had also been an increase in exception reporting (i.e. beta blockers reported as contraindicated/not indicated). • JS noted that we could also check whether hospitals have patients with above average levels of risk, which might indicate gaming in order to decrease risk-adjusted mortality rates. GBA noted that MINAP has a validation study, where hospitals are asked to resubmit records for 20 randomly selected patients, to ensure high quality data entry. The differences between the original record and the resubmitted record are used by MINAP as a measure of data quality. • JS noted that NICOR was hoping to roll out a more standardised system of validation across all of its audits, and would be taking examples of best practice from all projects in order to develop the best possible validation process. Action 5: PM to send details of BPT workshop to SH and TM. 5. Research update TM noted that she had discussed the impact award funding with Novartis. They had advised that NICOR would need to apply for a grant via the Novartis Corporate Responsibility project - applications are submitted via an electronic form, and would be reviewed by an independent group. JS noted that the application had already been written for UCL, so much of this could hopefully be reused. TM noted that Novartis are likely to be interested in this project, because of their new products relating to acute heart failure. Action 6: JS and PM to submit impact application to Novartis. 6. NICOR update JS informed the group that the next contract review meeting would be on 17 July. She noted that contract reviews had previously been quarterly and are now biannual, with all NICOR audits being reviewed on one day. PM confirmed that HF did not have any outstanding deliverables for April-July 2014. There are ongoing delays with ONS/HES linkage, due to an internal backlog at the HSCIC. NICOR has escalated the issue to HQIP, so they are aware that deliverables (e.g. report publication) may not be met in the agreed timeframe. PM noted that the HF annual report did not need to be published until November (prior to BSH annual meeting), so hopefully this would give enough leeway to accommodate the delay. NICOR is hiring an audit and research manager, two project managers, a data manager and two analysts. Adverts are currently online, and most close on 3 July. NICOR held a patient and public engagement day on 8 May, which was a success. A virtual patient and public panel is being developed, who will be able to advise NICOR on PPE issues, and had also found new patient representatives for several audit Steering Groups. HF patients, and the HF charity Pumping Marvellous, were also represented. JS attended a consultation with HQIP on outlier guidance. HQIP and DoH developed some outlier guidance in 2011, and NHS England has asked for this to be rewritten in August. It was noted that the original guidance had been based on the SCTS outlier policy, but it was not clear at this point what the new guidance would be based on. It will advise on outlier management for hospital and operator- level analysis. JS noted that the new guidance may require NICOR to go to CQC with potential outliers earlier in the outlier identification process that it has in the past, but that nothing would be acted upon until data had been validated. PM noted that HF does not yet have risk-adjusted analysis, and sought confirmation that the guidance would only apply to analysis that had been risk adjusted. JS confirmed that the guidance would also apply to process measures, as well as outcome measures, but was unclear whether the guidance would be a very specific set of rules or more general set of principles to consider in identifying outliers. Action 7: JS to inform clinical leads when further information about HQIP outlier policy is circulated. TM explained that many of the procedure-based audits (e.g. cardiac surgery, PCI, CRM) were now publishing at operator level, and asked whether pressure will be put on HF to follow suit. She noted that HF patients are often treated across the hospital, and by various members of the HF MDT, but also said that the HF clinical lead in a hospital does have some responsibility for the overall care of HF patients, even if they don’t personally see them all. RM said that he was more interested in the performance of the team than of individual members. TM commented that the make-up of the team, together with overall team performance and variation, might be more useful to focus on. PM noted that operator level analysis was not part of the HF 2014-16 deliverables, although we had agreed to potentially publishing a named clinical lead alongside hospital level data, with the proviso that they would be considered to have overall responsibility for audit data quality/completeness, and not necessarily total responsibility for the treatment and management of all HF patients in their hospital. RM agreed that it was important to have one named individual, to encourage hospitals to engage with their data. 7. NICOR publication processes TM reported that JS had sent a new publication procedure to all clinical leads, and that this should be shared with the Steering Group, as it potentially affected everyone. In summary: • Any non-contracted outputs from the audits, and other outputs where you state your affiliation to the audit, NICOR or UCL (e.g. letters, articles, presentations, abstract submissions, book chapters, conference proceedings) need to be approved by the NICOR Executive and HQIP. • If you are undertaking any work in a private capacity that includes any content related to NICOR or UCL or information that you are only privy to in relation to your employment/role at NICOR, then you must include the following disclaimer: “The views and opinions expressed in this paper are the author’s own personal views and do not necessarily represent or reflect the views and opinions of NICOR or UCL. For the avoidance of doubt NICOR or UCL has not supported or reviewed the author’s work in regards to this work”. • Conflicts of interest should be forwarded to Julie. It was raised as a concern that as part of the HQIP standard reporting procedure (SRP) you are supposed to inform them of publications 6 months in advance. This would not be possible in the case of many outputs. JS confirmed that HQIP were being reasonable, and would not delay publication. They had responded to congenital requests submitted less than two weeks before publication. JS noted that any projects and publications which had gone through the research process would be dealt with by a separate procedure. Action 8: TM will feed back the HF Steering Group’s thoughts on the publication process to the PLG on 16 July. 8. Changes to audit project management PM is starting a PhD in September and will thus be leaving the audit. She will continue to work at NICOR for one day per week, managing a new PROMs research project. TM thanked PM for her work on the audit. SH expressed concern that there would be a delay in recruiting a new project manager, and JS said that the adverts were closing on 4 July and interviews would be held over the next couple of weeks, so hopefully delays would be avoided. It was noted that project plans were in place, and the annual report would be outlined and drafted by the end of August, to make handover as smooth as possible. PM informed the group that since the beginning of June, HF had 60% FTE project management support, rather than 100%. 9. AOB • JA asked for more information on the prospective methodology pilot project. PM noted that at the previous meeting the inclusion criteria and timeline had been agreed. Around 10 sites would be identified, and the project would run for three months from September to November. It was noted that a comparison with HES was needed. It was suggested that participating sites collect all HES-coded HF patients (i.e. those meeting current audit criteria) and HF patients who have not been coded with HF in the primary position but nonetheless whose main reason for their hospital admission was heart failure. These could be distinguished using the ‘user defined fields’ in the dataset to record the clinical code given to the patient for their admission. Action 9: PM to circulate final criteria and details of prospective methodology audit. Action 10: PM to discuss with Marion Standing the best way of collecting pilot data (in same or separate database). • JA asked whether cardiac rehab could be added to hospital-level analyses in 2013/14 annual report; this was agreed. Action 11: PM to add referral cardiac rehab to hospital-level analyses in annual report analysis plan. • RM said that he had met someone who input data for the audit, and who found it very difficult to complete. All acknowledged that the audit is difficult to complete, because notes have to be pulled for each patient, and because of the depth of clinical data collected. KS noted that prospective identification saves them a lot of time at Kettering, as it means that the nurses can collect information on their ward rounds, and only a few pieces of additional information need to be picked up subsequently. It was noted that the implementation of the BPT would go some way to encouraging hospitals to put more administrative/clerical resource into the audit, as there would be a financial incentive attached to its completion. TM noted that currently non-participation triggers a CQC visit, which is motivation enough in many hospitals. Date and time of next meeting: Weds 15 October 2014, 13.30-15.30, NICOR boardroom Weds 7 January 2015, 13.30-15.30, NICOR boardroom NOTE CHANGE OF DATE Weds 15 April 2015, 13.30-15.30, NICOR boardroom Appendix: Action Points # Action Owner 1 2013/14 report to include slide pack with main analyses, and separate summary report. Project manager 2 JM and PM to investigate whether the Gloucestershire secondary care HF team is ready to start retrospective data collection. PM, JM 3 PM to add LOS against 30-day readmission/mortality (from discharge) to 2013/14 analysis plan. PM 4 Hospital level data to be sent to all HF contacts once ONS linkage has occurred. PM, AS, project manager 5 PM to send details of BPT workshop to SH and TM. PM 6 JS and PM to submit impact application to Novartis. PM, JS 7 JS to inform clinical leads when further information about HQIP outlier policy is circulated. JS 8 TM will feed back the HF Steering Group’s thoughts on the publication process to the PLG on 16 July. TM 9 PM to circulate final criteria and details of prospective methodology audit. PM 10 PM to discuss with Marion Standing the best way of collecting pilot PM, MS data (in same or separate database) 11 PM to add referral cardiac rehab to hospital-level analyses in annual report analysis plan. PM Completed?